| Company Name: |
Mainchem Co., Ltd.
|
| Tel: |
+86-0592-6210733 |
| Email: |
sale@mainchem.com |
| Products Intro: |
Product Name:CALCIUM PERMANGANATE
CAS:10118-76-0
|
| Company Name: |
3B Pharmachem (Wuhan) International Co.,Ltd.
|
| Tel: |
821-50328103-801 18930552037 |
| Email: |
3bsc@sina.com |
| Products Intro: |
Product Name:CALCIUM PERMANGANATE
CAS:10118-76-0
Purity:99% HPLC Package:1Mg ; 5Mg;10Mg ;100Mg;250Mg ;500Mg ;1g;2.5g ;5g ;10g
|
| Company Name: |
Triveni chemicals
|
| Tel: |
08048762458 |
| Email: |
sales@trivenichemical.com |
| Products Intro: |
Product Name:Calcium Permanganate
CAS:10118-76-0
|
|
| | CALCIUM PERMANGANATE Basic information |
| Product Name: | CALCIUM PERMANGANATE | | Synonyms: | acerdol;CAICIUM PERMANGANATE;kaliumpermanganat;permanganatedecalcium(french);permanganatocalcico;permanganicacid(hmno4),calciumsalt;CALCIUM PERMANGANATE;Dipermanganic acid calcium salt | | CAS: | 10118-76-0 | | MF: | CaMn2O8 | | MW: | 277.95 | | EINECS: | 233-322-7 | | Product Categories: | | | Mol File: | 10118-76-0.mol | 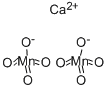 |
| | CALCIUM PERMANGANATE Chemical Properties |
| density | 2.400 | | solubility | reacts with ethanol | | form | purple hygroscopic crystals | | color | purp hygroscopic crystals, crystalline | | Water Solubility | very soluble H2O; decomposed by alcohol [MER06] | | EPA Substance Registry System | Permanganic acid (HMnO4), calcium salt (10118-76-0) |
| | CALCIUM PERMANGANATE Usage And Synthesis |
| Chemical Properties | violet or dark purple, deliquescent crystal(s); made by reacting KMnO4 and CaCl2; used in the textile industry, to sterilize water, and as a deodorizer [HAW93] [MER06] | | Uses | Antiseptic, disinfectant, deodorizer; with CaF2 as binder for welding electrode coatings and fluxes. | | General Description | Purple crystalline solid. CALCIUM PERMANGANATE is used as a disinfectant and deodorizer, in water purification, and for many other uses. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | CALCIUM PERMANGANATE is an oxidizing agent. Noncombustible but CALCIUM PERMANGANATE will accelerate the burning of combustible material. If the combustible material is finely divided the mixture may be explosive. Contact with liquid combustible materials may result in spontaneous ignition. Contact with sulfuric acid may cause fires or explosions. Mixtures with acetic acid or acetic anhydride can explode if not kept cold [Von Schwartz 1918 p. 34 ]. Explosions can occur when CALCIUM PERMANGANATE that has been treated with sulfuric acid comes in contact with benzene, carbon disulfide, diethyl ether, ethyl alcohol, petroleum, or other organic matter. | | Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Industrial uses | Calcium permanganate [Ca(MnO4)2] is used in textile
production, as a water-sterilising agent and in dental procedures. | | Safety Profile | Poison by intravenous
route. See also CALCIUM COMPOUNDS,
MANGANESE COMPOUNDS, and
PERMANGANATES. A strong oxidant.
May explode on contact with acetic acid or
acetic anhydride. Ignites on contact with
cellulose. Incompatible with hydrogen
peroxide. | | Purification Methods | Crystallise it from water (3.3mL/g) by partial evaporation in a desiccator. It is deliquescent. Note that it loses oxygen more readily than the potassium salt. It is an oxidising agent. |
| | CALCIUM PERMANGANATE Preparation Products And Raw materials |
|