- GLIOTOXIN
-

- $1.00 / 1g
-
2020-01-13
- CAS:67-99-2
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 200kg
|
| | GLIOTOXIN Basic information |
| Product Name: | GLIOTOXIN | | Synonyms: | GLIOTOXIN;GLIOTOXIN, GLADIOCLADIUM FIMBRIATUM;2,3,5A,6-TETRAHYDRO-6-HYDROXY-3(HYROXYMETHYL)-2-METHYL-10H-3A, 10A-EPIDITHIO-PYRAZINOL[1,2ALPHA]INDOLE-1,4-DIONE;(3r-(3-alpha,5a-beta,6-beta,10a-alpha))-ymethyl)-2-methyl;10h-3,10a-epidithiopyrazino(1,2-a)indole-1,4-dione,2,3,5a,6-hydroxy-3-(hydrox;aspergillin;s.n.12870;gliotoxin from gliocladium fimbriatum | | CAS: | 67-99-2 | | MF: | C13H14N2O4S2 | | MW: | 326.39 | | EINECS: | 636-170-3 | | Product Categories: | antibiotic | | Mol File: | 67-99-2.mol | 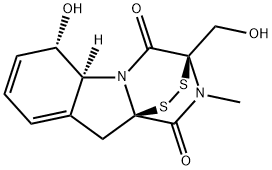 |
| | GLIOTOXIN Chemical Properties |
| Melting point | 221°C (rough estimate) | | Boiling point | 699.7±55.0 °C(Predicted) | | alpha | D25 -290° (c = 0.08 in ethanol) | | density | 1.4069 (rough estimate) | | refractive index | 1.6510 (estimate) | | Fp | 2℃ | | storage temp. | 2-8°C | | solubility | chloroform: 10 mg/mL, clear, colorless | | form | White solid | | pka | 12.90±0.40(Predicted) | | color | Monoclinic crystals from MeOH | | Merck | 13,4454 | | BRN | 50675 | | Stability: | Stable for 2 yeara as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| | GLIOTOXIN Usage And Synthesis |
| Description | Gliotoxin is an immunosuppressive mycotoxin produced by pathogenic strains of Aspergillus and other fungi with diverse biological activities. It inhibits 20S proteasomal chymotrypsin activity (IC50 = 10 μM), blocking the degradation of IκBα and preventing the activation of NF-κB. Gliotoxin induces apoptosis in monocytes and dendritic cells and reduces phagocytosis by neutrophils. It suppresses viral infection by Nipah and Hendra virus in HEK293T cells (IC50s = 149 and 579 nM, respectively). Under reducing conditions, gliotoxin inhibits leukotriene A4 hydrolase (LTA4H; ) epoxide hydrolase activity, but not aminopeptidase activity, and leukotriene B4 (LTB4; ) synthesis in neutrophils and monocytes. In vivo, gliotoxin (5 mg/kg) reduces LTB4 plasma levels and blocks peritoneal neutrophil infiltration in a mouse model of peritonitis induced by zymosan A . It also inhibits geranylgeranyltransferase I and farnesyltransferase (IC50s = 17 and 80 μM, respectively). | | Chemical Properties | White powder | | Uses | Gliotoxin is a potent epithiodioxopiperazine mycotoxin produced by species of Gliocladium, Aspergillus and Penicillium. At the cellular level gliotoxin inhibits a broad range of unrelated mechanisms, including inhibition of chymotrypsin-like activity of the 20S proteasome and Ca2+ release from mitochondria, activation of transcription factor NF-κB in response to a variety of stimuli in T and B cells, anti-inflammatory activity, and inhibition of farnesyltransferase and geranylgeranyltransferase. The mode of action appears to be via covalent interaction with proteins through mixed disulphide formation. Gliotoxin inhibits a number of thiol-requiring enzymes and also displays antioxidant and immunomodulatory activity. | | Uses | Gliotoxin is a sulfur-containing mycotoxin produced by species of fungi and pathogens of humans. Gliotoxin exhibits inhibitory activities against histone H3K9 methyltransferase, a key enzyme in the re
gulation of transcriptional activity by writing epigenetic marks. Gliotoxin also exhibits immunosuppressive properties by causing apoptosis of cells of the immune system. In addition, various studies
suggests Gliotoxin may also be a potential anti-inflammatory, antibiotic, antifungal and antiviral agent. | | Definition | ChEBI: A pyrazinoindole with a disulfide bridge spanning a dioxo-substituted pyrazine ring; mycotoxin produced by several species of fungi. | | Hazard | Poison. | | Biological Activity | Immunosuppressive agent; blocks phagocytosis, cytokine production and proliferation of T and B cells. Non-competitively inhibits chymotrypsin-like activity of 20S proteasome; prevents degradation of I κ B α , an endogenous blocker of NF- κ B. Also inhibits farnesyltransferase and geranylgeranyltransferase I (IC 50 values are 80 and 17 μ M respectively) and displays antitumor activity against breast cancer in vivo . | | Safety Profile | Poison by intraperitoneal andintravenous routes. When heated to decomposition itemits very toxic fumes such as SOx and NOx. | | target | COX | Wnt/β-catenin | Antifection | | storage | -20°C (desiccate) | | Purification Methods | Purify gliotoxin by recrystallisation from MeOH. Its solubility in CHCl3 is 1%. The dibenzoyl derivative has m 202o (from CHCl3/MeOH). [Glister & Williams Nature 153 651 1944, Elvidge & Spring J Chem Soc Suppl 135 1949, Johnson et al. J Am Chem Soc 65 2005 1943, Bracken & Raistrick Biochem J 41 569 1947.] | | References | Waring & Beaver (1996), Gliotoxin and related epipolythiodioxopiperazines; Gen. Pharmacol., 27 1311
Kroll et al. (1999), The secondary fungal metabolite gliotoxin targets proteolytic activities of the proteasome; Chem. Biol., 6 689
Fitzpatrick et al. (2000), In vitro and in vivo effects of gliotoxin, a fungal metabolite: efficacy against dextran sodium sulfate-induced colitis in rats; Dig. Dis. Sci., 45 2327
Konig et al. (2019), Gliotoxin from Aspergillus fumigatus Abrogates Leukotriene B4 Formation through Inhibition of Leukotriene A4 Hydrolase ; Cell Chem. Biol., 26 524
Hubmann et al. (2020), Targeting Nuclear NOTCH2 by Gliotoxin Recovers a Tumor-Suppressor NOTCH3 Activity in CLL; Cells, 9 1484 |
| | GLIOTOXIN Preparation Products And Raw materials |
|