- ecteinascidin 743
-

- $30.00 / 1kg
-
2023-11-24
- CAS:114899-77-3
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20 tons
- Trabectedin
-

- $0.00 / 1MG
-
2023-02-07
- CAS:114899-77-3
- Min. Order: 1MG
- Purity: 98%min
- Supply Ability: 30kg/month
- ecteinascidin 743
-

- $2.00 / 1kg
-
2019-07-06
- CAS:114899-77-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100kg
|
| | ecteinascidin 743 Basic information |
| Product Name: | ecteinascidin 743 | | Synonyms: | ecteinascidin 743;(1'R,6R,6aR,7R,13S,14S,16R)-;Trabectedin(Ecteinascidin 743,NSC-684766,ET-743, Yondelis);Ecteinascidine 743;NSC 648766;Trabectedin(Ecteinascidin 743,NSC-684766,ET-743);Trabectedin
Ecteinascidin;Yondelis R ,Trabectedin | | CAS: | 114899-77-3 | | MF: | C39H43N3O11S | | MW: | 761.84 | | EINECS: | | | Product Categories: | Amines;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;API;114899-77-3 | | Mol File: | 114899-77-3.mol | 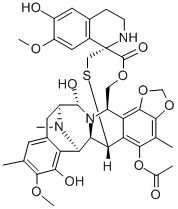 |
| | ecteinascidin 743 Chemical Properties |
| Melting point | >143°C (dec.) | | alpha | D25 +114° (c = 0.1 in methanol) | | density | 1.55±0.1 g/cm3 (20 ºC 760 Torr) | | storage temp. | -20°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 9.73±0.40(Predicted) | | form | Solid | | color | Light Yellow to Yellow | | InChIKey | PKVRCIRHQMSYJX-HAHYLZASNA-N |
| | ecteinascidin 743 Usage And Synthesis |
| Description | Trabectedin is a marine natural product derived from the tunicate
Ecteinascidia turbinate. With its demonstrated in vitro and in vivo activity against
a range of solid tumor cell lines, human xenografts and tumor explants, this
antineoplastic agent has been developed and launched for the treatment of
advanced STS after failure of first-line therapy with anthracyclines or ifosfamide
or in patients who are unsuited to receive these agents. Its proposed mechanism
of action involves binding to the N2 position of guanine in the minor groove
demonstrating a preference for sequences containing 5′-PuGC and 5′-PyGG
motifs. Subsequent alkylation of DNA, via an iminium intermediate generated
from an intra-molecular acid-catalyzed activation and dehydration of the
carbinolamine, induces a curvature of the DNA toward the major groove that
ultimately disrupts the binding of transcription factors involved in cell
proliferation. Evaluation of trabectedin against the National Cancer Institute’s
human in vitro cell line panel, including melanoma, non-small-cell lung, ovarian,
renal, prostate, and breast cancer, demonstrated potencies ranging from 1 pM to
10 nM. | | Originator | University of Illinois (US) | | Uses | A tetrahydroisoquinoline alkaloid with antitumor activity isolated from the Caribbean tunicate, Ecteinascidin turbinata. It is the first marine anticancer agent approved in the European Union for patients with soft tissue sarcoma (STS). Antineoplastic. | | Definition | ChEBI: A tetrahydroisoquinoline alkaloid obtained from a Caribbean tunicate Ecteinascidia turbinata. Used for the treatment of soft tissue sarcoma and relapsed ovarian cancer. | | Brand name | Yondelis | | Clinical Use | Antineoplastic agent:
Advanced soft tissue sarcoma
Ovarian cancer | | Side effects | The most common adverse events included nausea, fatigue, vomiting, anorexia, neutropenia, and increases in aspartate aminotransferase and alanine aminotransferase liver enzymes. To combat the nausea and vomiting, all patients must receive 20mg of dexamethasone intravenously before the trabectedin infusion. Not only does this pretreatment have an antiemetic effect, it also appears to offer a hepatoprotective benefit. Concomitant administration of potent inhibitors and inducers of CYP3A4 should be avoided since plasma levels of this CYP3A4- metabolized drug will be affected. Trabectedin is contraindicated in patients with a known hypersensitivity to trabectedin, concurrent serious or uncontrolled infection, or breast-feeding. Trabectedin should also not be administered in combination with yellow fever vaccine. Finally, there are strict criteria regarding absolute neutrophil count, platelet count, and renal and hepatic enzyme levels for permission to initiate or continue treatment with trabectedin. | | Synthesis | While the challenging total synthesis of trabectedin has been accomplished by a few research groups, the commercial preparation begins from readily available cyanosafracin B in 21 steps. Following the Boc and MOM protection of the amine and phenol functionalities, respectively, the methoxy-pquinone was hydrolyzed with sodium hydroxide in methanol. The resulting quinine was reduced (hydrogen over Pd/C) to give an unstable hydroquinone that was subsequently alkylated with bromochloromethane and allyl bromide. The MOM and Boc groups were then removed followed by cleavage of the amide by Edman degradation (through formation of the thiourea with phenyl isothiocyanate and treatment with HCl in 1,4-dioxane). At this point, the amine was protected as the TROC carbamate before reprotecting the phenol as the MOM ether and then liberating the amine with zinc in acetic acid. The amine was converted to an alcohol moiety with sodium nitrite in acetic acid, and this handle was acylated with (S)-N-[(trichloroethoxy)carbonyl]-S-(9-fluorenylmethyl)cysteine. Removal of the allyl-protecting group followed by oxidation provided an 492 Shridhar Hegde and Michelle Schmidt alpha-hydroxy ketone intermediate. Dehydration and deprotection of the cysteine established the Michael addition of the thiol to the o-quinone methide with concomitant trapping with acetic anhydride. The remaining steps involved removal of protecting groups, installation of the tetrahydroisoquinoline ring via a Pictet Spengler reaction, and conversion of the cyano to the alcohol of the carbinolamine with silver nitrate in acetonitrile and water. | | Drug interactions | Potentially hazardous interactions with other drugs
Alcohol: Avoid concomitant use.
Antibacterials: concentration reduced by rifampicin.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Vaccines: risk of generalised infections - avoid. | | Metabolism | Metabolised in the liver, mainly by cytochrome P450 isoenzyme CYP3A4. Excreted mainly via the faeces. |
| | ecteinascidin 743 Preparation Products And Raw materials |
|