- Amantadine
-
- $50.00 / 1kg
-
2024-04-23
- CAS:768-94-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 8000kg
- Amantadine
-
- $32.00 / 1kg
-
2024-04-07
- CAS:768-94-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Amantadine
-

- $25.00 / 1kg
-
2023-08-22
- CAS:768-94-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100tons
|
| | Amantadine Chemical Properties |
| Melting point | 206-208 °C(lit.) | | Boiling point | 263.29°C (rough estimate) | | density | 0.9510 (rough estimate) | | refractive index | 1.5220 (estimate) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | 1 M HCl: soluble5%, clear to hazy, colorless to faint yellow or tan | | form | Powder | | pka | 10.1(at 25℃) | | color | White to cream | | Water Solubility | Soluble in organic solvents. Insoluble in water. | | Merck | 14,374 | | BRN | 2204333 | | InChIKey | DKNWSYNQZKUICI-UHFFFAOYSA-N | | CAS DataBase Reference | 768-94-5(CAS DataBase Reference) | | NIST Chemistry Reference | Tricyclo[3.3.1.13,7]decane-1-amine(768-94-5) | | EPA Substance Registry System | Tricyclo[3.3.1.13,7]decan-1-amine (768-94-5) |
| | Amantadine Usage And Synthesis |
| Inhibitor | The anti-influenza A compound amantadine acts by blocking the M2 ion channel which is required for uptake of protons into the interior of the virus to permit acid-promoted viral uncoating (decapsidation).
| | Description | Amantadine is an agent that raises the concentration of dopamine in the synaptic cleft by
releasing it from neurons and suppressing the process of reuptake. | | Chemical Properties | White to cream powder | | Uses | Amantadine is a primary amine derivative of adamantane. It has an effect on mycoviruses,
which are RNA-containing viruses. It has a very narrow spectrum of action and is used only for
treating and preventing influenza A. It is also used for treating Parkinsonism. The exact mech�anism of antiviral action of amantadine is not completely understood. It is believed that it is an
ion channel blocker. It has also been suggested that amantadine inhibits absorption of viral par�ticles into the host cell, which is expressed in the breakdown of diffusion of the virus into the
cell, or inhibition of the “stripping process” of the virus. The main use is for the prevention of
type A2 influenza. Synonyms of this drug are simmetrel, viregit, mantadan, and others. | | Uses | Building block for an L-piperidinamide catalyst used in an enantioselective Strecker reaction of phosphinoyl imines.1 | | Uses | Amantadine is an antivi�ral drug. The properties in amantadine, which relieve symptoms of Parkinsonism were dis�covered by accident. Treatment of Parkinsonism with a combination of levodopa,
anticholinergic drugs, and amantadine gives better results than using any of these drugs
individually. | | Definition | ChEBI: A member of the class of adamantanes that is used as an antiviral and antiparkinson drug. | | Brand name | Symadine (Solvay Pharmaceuticals); Symmetrel
(Endo). | | Biological Functions | Amantadine was originally introduced as an antiviral
compound, but it is modestly effective
in treating symptoms of parkinsonism. It is useful in the
early stages of parkinsonism or as an adjunct to levodopa
therapy. Its mechanism of action in parkinsonism
is not clear, but amantadine may affect dopamine
release and reuptake. Additional sites of action may
include antagonism at muscarinic and N-methyl-Daspartate
(NMDA) receptors. Adverse effects include
nausea, dizziness, insomnia, confusion, hallucinations,
ankle edema, and livedo reticularis. Amantadine and
the anticholinergics may exert additive effects on mental
functioning | | Synthesis Reference(s) | Journal of the American Chemical Society, 91, p. 6457, 1969 DOI: 10.1021/ja01051a047
Synthesis, p. 457, 1976 | | Antimicrobial activity | It inhibits influenza A virus replication at concentrations of
0.2–
0.6 mg/L, but has little or no activity against influenza
B or C. | | Acquired resistance | Resistance is the consequence of mutations in amino acid
positions 27, 30 and 31 in the M2 transmembrane sequence.
Cross-resistance between amantadine and rimantadine is universal.
Influenza H3N2 strains worldwide are now resistant,
but seasonal H1N1 strains remain susceptible. Postexposure
family prophylaxis results in the prompt emergence of drug
resistance after onset of treatment. | | General Description | Amantadine has been used for years as a treatment for Parkinson disease. The adamantanamines have twomechanisms in common:they inhibit an early step in viralreplication, most likely viral uncoating,and in somestrains, they affect a later step that probably involves viral assembly,possibly by interfering with hemagglutinin processing.The main biochemical locus of action is the influenzatype A virus M2 protein, which is an integral membrane proteinthat functions as an ion channel. The M2 channel is a protontransport system. By interfering with the function of theM2 protein, the adamantanamines inhibit acid-mediated dissociationof the ribonucleoprotein complex early in replication.They also interfere with transmembrane proton pumping,maintaining a high intracellular proton concentrationrelative to the extracellular concentration and enhancingacidic pH-induced conformational changes in the hemagglutininduring its intracellular transport at a later stage. The conformationalchanges in hemagglutinin prevent transfer of thenascent virus particles to the cell membrane for exocytosis. | | Flammability and Explosibility | Flammable | | Pharmaceutical Applications | A symmetrical synthetic C-10 tricyclic amine with an unusual
cage-like structure, supplied as the hydrochloride for oral
administration. | | Mechanism of action | Amantadine hydrochloride (1-adamantanamine hydrochloride) is a symmetric, tricyclic, primary amine that
inhibits penetration of RNA viral particles into the host cell. It also inhibits the early stages of viral
replication by blocking the uncoating of the viral genome and the transfer of nucleic acid into the host
cell. | | Pharmacokinetics | Oral absorption: >90%
Cmax 200 mg oral per day: 0.4–0.9 mg/L after c. 4–6 h
Plasma half-life: 9.7–14.5 h
Volume of distribution: 10.4 L/kg
Plasma protein binding: 65%
Absorption and distribution
Absorption after oral administration is almost complete. Levels in secretions approach plasma concentrations.
Metabolism and excretion
About 56% of a single oral dose is excreted unchanged within 24 h by the kidney. Altogether 90% of an oral dose is excreted in the urine with a mean elimination half-life of 11.8 h in subjects with normal renal function. In elderly men, the half-life is 28.9 h and in patients with renal insufficiency half-lives of 18.5 h to 33.8 days have been observed. The renal clearance is around 398 mL/min (range 112–772 mL/min), indicating active secretion as well as glomerular filtration. Less than 5% of a dose is removed during hemodialysis and average half-lives of 8.3 and 13 days have been reported in patients on chronic hemodialysis. Extreme care must be taken to ensure that drug does not accumulate to toxic levels. | | Clinical Use | Amantadine is used for the treatment
of diseases caused by influenza A strains. | | Clinical Use | Prevention and treatment of influenza A H1N1 infections | | Side effects | Embryotoxicity and teratogenicity have been observed in rats
receiving 50 mg/kg per day, about 15 times the usual human
dose. Neurological side effects include drowsiness, insomnia,
light-headedness, difficulty in concentration, nervousness, dizziness
and headache in up to 20% of individuals. Other side
effects include anorexia, nausea, vomiting, dry mouth, constipation
and urinary retention. All develop during the first
3–4 days of therapy and are reversible by discontinuing the
drug. An exception to rapid onset of adverse reactions is livedo
reticularis. Convulsions, hallucinations and confusion are dose
related, usually occurring at levels in excess of 1.5 mg/L; convulsions
may occur at a lower threshold in patients with a history
of epilepsy and the drug is best avoided in such patients. | | Safety Profile | Poison by intraperitoneal route.Moderately toxic by ingestion. Mutation data reported.When heated to decomposition it emits toxic fumes ofNOx. Used as an antiviral agent. | | Synthesis | Amantadine, 1-adamantanamine (10.1.12), is synthesized from adaman�tane. It is directly brominated to 1-bromadamantane (10.1.10), which in Ritter reaction
conditions when heated with a mixture of acetonitrile and concentrated sulfuric acid trans�forms into 1-acetylaminoadamantane (10.1.11). Hydrolysis of this product using alkali
leads to the formation of amantadine (10.1.12) [16,17]. 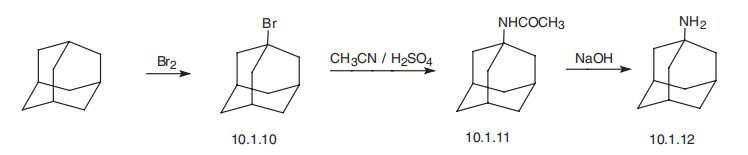
| | Purification Methods | Dissolve the amine in Et2O, dry it over KOH, evaporate and sublime it in vacuo. [Stetter et al. Chem Ber 93 226 1960.] |
| | Amantadine Preparation Products And Raw materials |
|