| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
Product Name:Halowax 1013
CAS:1321-64-8
Purity:0.1 Mg/ML in MeOH Package:1ML
|
|
| | HALOWAX 1013 Basic information |
| Product Name: | HALOWAX 1013 | | Synonyms: | chlorinatednaphthalenes;pentachloro-naphthalen;pentachloronaphthalene;Naphthalene, pentachloro-;Polychlorierte Naphthaline;Pentachloronapthalene;penthachloronaphthalene;Halowax 1013@0.1 mg/mL in MeOH | | CAS: | 1321-64-8 | | MF: | C10H3Cl5 | | MW: | 300.39582 | | EINECS: | 215-320-8 | | Product Categories: | | | Mol File: | 1321-64-8.mol | 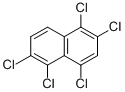 |
| | HALOWAX 1013 Chemical Properties |
| Melting point | 120°C | | Boiling point | 384.34°C (rough estimate) | | density | 1.7000 | | refractive index | 1.5538 (estimate) | | form | solid | | color | White | | EPA Substance Registry System | Pentachloronaphthalene (1321-64-8) |
| | HALOWAX 1013 Usage And Synthesis |
| Chemical Properties | Pentachloronaphthalene is a pale yellow or
white solid powder with an aromatic odor. | | Chemical Properties | The chlorinated naphthalenes in which one or
more hydrogen atoms have been replaced by chlorine to
form wax-like substances, beginning with monochloronaphthalene and going on to the octachlor derivatives.
Their physical states vary from mobile liquids to waxysolids depending on the degree of chlorination; freezing/
melting points of the pure compounds range from 17C
for 1-chloronaphthalene to 198C for 1,2,3,4-
tetrachloronaphthalene.
1-Chloro-isomer: Hazard identification (based on
NFPA-704 M Rating System): Health 2, flammability 1,
reactivity 0.
2-Chloro-isomer: | | Uses | In electric wire insulation; in lubricants | | General Description | Pale-yellow to white solid or powder with an aromatic odor. Bp: 327-371°C; mp: 120°C. Density 1.7 g cm-3. Used in lubricants and in the manufacture of insulation for electrical wire. Presents an environmental danger. If released into the environment, bioaccumulation takes place, specifically in fish. Will persist in the environment causing long-term adverse effects. The halowaxes are technical-grade chlorinated naphthalenes containing HALOWAX 1013 in its various isomers together with (mainly) trichloro- tetrachloro- and hexa-chloronapthalenes in their various isomers. | | Reactivity Profile | HALOWAX 1013 is non-flammable, but combustible. Gives off irritating or toxic gases in a fire. Incompatible with strong oxidizing agents. Reacts violently with aluminum, with bases, and with liquid O2. | | Health Hazard | Pentachloronaphthalene is toxic
to the liver and skin. | | Safety Profile | Poison by ingestion, inhalation, and skin contact. An irritant. Action similar to that of chlorinated naphthalenes and chlorinated diphenyls. Dangerous; when heated to decomposition it emits highly toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, AROMATIC | | Potential Exposure | Used in electric wire insulation, in
additives to specialized lubricants; and as a fire-and waterproofing
agent. No longer produced or used in the US. | | Shipping | UN3082 Environmentally hazardous substances,
liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. | | Incompatibilities | Violent reaction with strong oxidizers,
aluminum, liquid oxygen; potassium, sodium. Heat may
contributes to instability. | | Waste Disposal | High-temperature incineration
with flue gas scrubbing. Incineration, preferably after mixing with another combustible fuel. Care must be exercised
to assure complete combustion to prevent the formation of
phosgene. An acid scrubber is necessary to remove the halo
acids produced. |
| | HALOWAX 1013 Preparation Products And Raw materials |
|