Cevimelinehydrochloride manufacturers
- Cevimelinehydrochloride
-

- $8.80 / 1KG
-
2019-07-06
- CAS:153504-70-2
- Min. Order: 1KG
- Purity: 97%-99%
- Supply Ability: 100kg
|
| | Cevimelinehydrochloride Basic information |
| Product Name: | Cevimelinehydrochloride | | Synonyms: | (2R,5R)-2-Methylspiro[1,3-oxathiolane-5,8'-1-azabicyclo[2.2.2]octane] hydrate dihydrochloride;CeviMeline hydrochloride heMihydrates;Cevimeline HCl 1/2H2o;cis-2-Methylspiro(1,3-oxathiolane-5,3')quinuclidine hydrochloride hydrate (2:2:1);Unii-p81Q6V85np;Cevimeline HCl hemihydrate;CevimelineHCl;Cevimelinehydrochloride | | CAS: | 153504-70-2 | | MF: | C10H20ClNO2S | | MW: | 253.79 | | EINECS: | | | Product Categories: | Inhibitors | | Mol File: | 153504-70-2.mol | 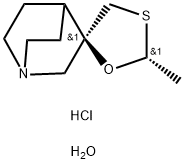 |
| | Cevimelinehydrochloride Chemical Properties |
| storage temp. | -20°C | | solubility | H2O: ≥25mg/mL | | form | powder | | color | white to tan | | InChIKey | ZSTLCHCDLIUXJE-MMPAUJBDSA-N |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN 2811 6.1 / PGIII | | WGK Germany | 3 |
| | Cevimelinehydrochloride Usage And Synthesis |
| Description | Although it was initially developed as a cognition enhancer, cevimeline was launched in
the US for the treatment of dry mouth symptoms (xerostomia) in patients with Sjogren’s
syndrome. Cevimeline is a racemic mixture of cis-oxathiolanes that can be obtained with a
three step synthesis starting from quinuclidin-3-one followed by separation from its 9-12-
fold less potent trans-diastereomer, This conformationally rigid analog of acetylcholine is a dual muscarinic M1/M3 agonist, selective versus M2, M4 and M5 receptors. It is the fifth M,
agonist that has failed in clinical trials against Alzheimer’s disease. On the contrary, the
sialagogic effects of cevimeline caused by its stimulation of M3 receptors in salivary and
lacrimal glands were demonstrated in randomized double-blind placebo-controlled clinical
trials (30 mg t.i.d. oral dose). Cevimeline (l-3 mg/kg i.v.) was as potent in dogs as
pilocarpine (0.1-0.3 mg/kg i.v.), the only prior drug efficacious against xerostomia
associated with Sjogren’s syndrome, but the effects of cevimeline lasted around 2-fold
longer. No cardiovascular side effects were reported with cevimeline, unlike pilocarpine
which has a 40-fold higher affinrty for the M2 receptor. Cevimeline seems to bind
extensively to tissues (volume of distribution 6 L/kg in man) since it was found to be less
than 20% bound to human plasma proteins. It is metabolized into the cis and transsulfoxide,
a glucuronide conjugate and the N-oxide. | | Originator | Israel Institute for Biological Research/Snow Brand (Israel) | | Uses | Treatment of
cancer. | | Uses | Cevimeline hydrochloride hemihydrate may be used in cell signaling studies. | | Manufacturing Process | (1) Into a 500 ml four-necked flask equipped with a stirrer, a thermometer
and a calcium chloride tube, 10.6 g of 3-hydroxy-3-
mercaptomethylquinuclidine (purity: 98.3%), 222.8 g of chloroform, 31.7 g of
toluene and 2.2 g of dimethylsulfoxide were charged, and 17.3 g of
acetaldehyde was added thereto at 10-15°C. While maintaining the
temperature at the same level, 12.2 g of anhydrous sodium sulfate was added
thereto. Then, 9.8 g of hydrogen chloride gas was blown thereinto over a
period of two hours, and then the mixture was maintained at room
temperature for 6 hours with stirring.
To the reaction mixture, 125.7 g of a 15% sodium hydroxide aqueous solution
was dropwise added to make the reaction mixture strongly alkaline. Then,
undissolved inorganic salts were separated by filtration, and the inorganic salts were washed with 18.9 g of chloroform. The filtrate was subjected to
liquid separation, and the aqueous layer was re-extracted with chloroform.
These chloroform layers were put together, and 100.5 g of 5% sulfuric acid
was added thereto to obtain desired 2-methylspiro(1,3-oxathiolane-
5,3')quinuclidine sulfate. Then, it was again made alkaline with 54.6 g of a
10% sodium hydroxide aqueous solution to free the desired 2-
methylspiro(1,3-oxathiolane-5,3')quinuclidine, which was then extracted four
times with 33 g of n-hexane. The n-hexane layer was dried over anhydrous
sodium sulfate, and then, sodium sulfate was filtered off to obtain a n-hexane
solution of 2-methylspiro(1,3-oxathiolane-5,3')quinuclidine. To this n-hexane
solution, 18.0 g of an iso-propyl alcohol solution containing 20% of
hydrochloric acid was dropwise added to obtain 2-methylspiro(1,3-
oxathiolane-5,3')quinuclidine hydrochloride. After stirring for 3 hours,
precipitated white crystals were collected by filtration to obtain 10.1 g of a
mixture of hydrochlorides of trans- and cis-2-methylspiro(1,3-oxathiolane-
5,3')quinuclidine (purity: 95.8%, yield of pure product 68.5%).
(2) Into a 500 ml four-necked flask equipped with a stirrer and a
thermometer, 4.9 g (0.02 mol) of the mixture of hydrochlorides of trans- and
cis-2-methylspiro(1,3-oxathiolane-5,3')quinuclidine obtained in the above step
(1) (the weight ratio of cis-and trans-isomers was 50.5/49.5) and 34 ml of
chloroform (this chloroform contained 0.5 wt % of ethyl alcohol) were
charged, and 17 ml of a chloroform solution containing 0.2 g of hydrogen
chloride (this chloroform also contained 0.5 wt % of ethyl alcohol) was added
thereto with stirring. Then, 7.8 g of stannic chloride was dropwise added
thereto over a period of 30 min, and an isomerization reaction was carried out
with stirring at room temperature for 24 hours. To the reaction product, 50 ml
of water was added, and a 48% sodium hydroxide aqueous solution was
added thereto with stirring to make the reaction mixture strongly alkaline.
Then, the chloroform layer was separated. To the aqueous solution, 10 ml of
chloroform was added for re-extraction. These chloroform layers were put
together, and 24.0 g of 5% sulfuric acid was added thereto to convert 2-
methylspiro(1,3-oxathiolane-5,3')quinuclidine in the reaction mixture to a
sulfate, which was then dissolved in water. To this aqueous layer, a 10%
sodium hydroxide aqueous solution was again added to make it strongly
alkaline and to free 2-methylspiro(1,3-oxathiolane-5,3')quinuclidine in the
reaction mixture. Then, it was extracted four times with 15 ml of n-hexane.
The extracted n-hexane layer was dried over anhydrous sodium sulfate. Then,
an iso-propyl alcohol solution containing 20% of hydrochloric acid was
dropwise added thereto to convert 2-methylspiro(1,3-oxathiolane-
5,3')quinuclidine in the reaction mixture to a hydrochloride, and precipitated
white crystals were collected by filtration and dried to obtain 4.4 g of a
mixture containing hydrochlorides of cis- and trans-2-methylspiro(1,3-
oxathiolane-5,3')quinuclidine (yield hydrochlorides: 92.1%). The ratio of cis-and trans-2-methylspiro(1,3-oxathiolane-5,3')quinuclidine was 98.6/1.4 (was
analyzed by liquid chromatography). | | Brand name | Evoxac | | Therapeutic Function | Salivation stimulant | | General Description | Cevimeline (Evoxac) iscis-2 -methylspiro {1-azabicyclo [2.2.2] octane-3, 5' -[1,3]oxathiolane} hydrochloride, hydrate (2:1). Cevimeline has amolecular weight of 244.79 and is a white to off white crystallinepowder. It is freely soluble in alcohol, chloroform, andwater. Cevimeline is a cholinergic agonist which binds to theM3 muscarinic receptor subtype, which results in an increasesecretion of exocrine glands, such as salivary and sweatglands. Because of these effects, it was approved for use in thetreatment of dry mouth associated with Sj?gren syndrome. Bystimulating the salivary muscarinic receptors cevimeline promotessecretion thereby alleviating dry-mouth in these patients.Cevimeline is metabolized by the isozymes CYP2D6and CYP3A3 and CYP3A4. It has a half-life of 5 hours. | | Biochem/physiol Actions | Cevimeline stimulates the peripheral muscarinic acetylcholine receptors of salivary glands and increases the concentration of Ca+2 in parotic acini and duct cells of rats. It thus acts as therapeutic agent for xerostomia. | | references | [1]. iga y, arisawa h, ogane n, et al. (+/-)-cis-2-methylspiro[1,3-oxathiolane-5,3'-quinuclidine] hydrochloride, hemihydrate (sni-2011, cevimeline hydrochloride) induces saliva and tear secretions in rats and mice: the role of muscarinic acetylcholine receptors. jpn j pharmacol, 1998, 78(3): 373-380.
[2]. fisher a, brandeis r, karton i, et al. (+-)-cis-2-methyl-spiro(1,3-oxathiolane-5,3') quinuclidine, an m1 selective cholinergic agonist, attenuates cognitive dysfunctions in an animal model of alzheimer's disease. j pharmacol exp ther, 1991, 257(1): 392-403. |
| | Cevimelinehydrochloride Preparation Products And Raw materials |
|