|
|
| | 5-bromo-1,3-dimethyl-1H-indazole Basic information |
| Product Name: | 5-bromo-1,3-dimethyl-1H-indazole | | Synonyms: | 5-bromo-1,3-dimethyl-1H-indazole;5-Bromo-1,3-dimethylindazole;5-BroMo-1,3-diMethylindaz...;3-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazol-2(3H)-one;1H-Indazole, 5-bromo-1,3-dimethyl- | | CAS: | 552331-30-3 | | MF: | C9H9BrN2 | | MW: | 225.09 | | EINECS: | | | Product Categories: | | | Mol File: | 552331-30-3.mol | 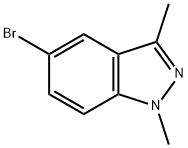 |
| | 5-bromo-1,3-dimethyl-1H-indazole Chemical Properties |
| storage temp. | 2-8°C | | Appearance | Off-white to light yellow Solid |
| | 5-bromo-1,3-dimethyl-1H-indazole Usage And Synthesis |
| Synthesis | Example 112A Synthesis of 5-bromo-1,3-dimethyl-1H-indazole: 5-bromo-3-methyl-1H-indazole (500 mg; 2.37 mmol) was added to a suspension of 60% sodium hydride (115 mg; 2.84 mmol) in N,N-dimethylformamide (10 mL). After stirring for 15 min at room temperature, iodomethane (456 mg; 3.21 mmol) was added and the reaction was continued with stirring for 2 hours. Upon completion of the reaction, the reaction mixture was diluted with water and extracted with ethyl acetate. The organic phases were combined, washed sequentially with water and saturated brine, and dried over anhydrous magnesium sulfate. The organic phases were concentrated under reduced pressure and the resulting crude product was purified by fast column chromatography (eluent: ether/hexane=1:1) to afford the target compound 5-bromo-1,3-dimethyl-1H-indazole (360 mg; 67% yield). | | References | [1] Bioorganic and Medicinal Chemistry, 2006, vol. 14, # 20, p. 6832 - 6846
[2] Patent: US2003/187026, 2003, A1
[3] Patent: US2003/199511, 2003, A1. Location in patent: Page/Page column 41
[4] Patent: WO2017/55305, 2017, A1. Location in patent: Page/Page column 53
[5] Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 11, p. 2504 - 2518 |
| | 5-bromo-1,3-dimethyl-1H-indazole Preparation Products And Raw materials |
|