|
|
| | Hapepunine Basic information |
| Product Name: | Hapepunine | | Synonyms: | Hapepunine;N-Methyl-(22S,25S)-dihydrotomatidenol;Pregn-5-ene-3,16-diol, 20-[(2S,5S)-1,5-dimethyl-2-piperidinyl]-, (3β,16β,20S)- (9CI);Pregn-5-ene-3,16-diol, 20-[(2S,5S)-1,5-dimethyl-2-piperidinyl]-, (3β,16β,20S)- | | CAS: | 68422-01-5 | | MF: | C28H47NO2 | | MW: | 429.69 | | EINECS: | | | Product Categories: | Alkaloids | | Mol File: | 68422-01-5.mol | 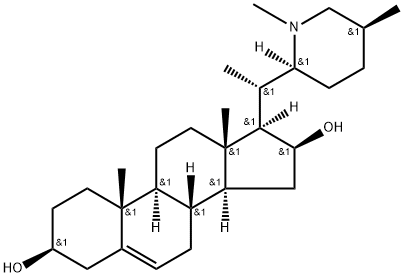 |
| | Hapepunine Chemical Properties |
| Boiling point | 540.4±40.0 °C(Predicted) | | density | 1.09±0.1 g/cm3(Predicted) | | pka | 15.01±0.70(Predicted) |
| | Hapepunine Usage And Synthesis |
| Occurrence | A steroidal alkaloid, hapepunine has been obtained by hydrolysis of the glycosidic fraction
of the extract from the aerial parts of Fritillaria carntschatcensis. It yields colourless needles when crystallized from EtOH and has a specific rotation of [α]D -72.6° (c 0.23,
CHCI3). The base has been characterized as the diacetate with m.p. 207-212°C. | | Uses | Hapepunine is a N-methyl-22,26-epiminochole stene, that can be isaolated from the aerial parts of Fritillaria camtschatcensis[1]. | | References | [1] Kaneko K, et al. Two steroidal alkaloids, hapepunine and anrakorinine, from the mature Fritillaria camtschatcensis. Phytochemistry, 1981, 20(1):157-160. |
| | Hapepunine Preparation Products And Raw materials |
|