- Xylitol
-
-
2025-11-30
- CAS:87-99-0
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Xylitol
-

- $0.00 / 25KG
-
2025-11-29
- CAS:87-99-0
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Xylitol
-

- $0.00 / 10kg
-
2025-11-28
- CAS:87-99-0
- Min. Order: 0.001kg
- Purity: 99.99%
- Supply Ability: 1000000kg
Related articles - Health benefits of Xylitol
- Xylitol is a sugar alcohol which provides 100% of the sugar sweetness of sucrose and has been shown to inhibit growth of some ....
- Mar 9,2022
|
| | Xylitol Basic information |
| | Xylitol Chemical Properties |
| Melting point | 94-97 °C (lit.) | | Boiling point | 215~217℃ | | density | 1.515 | | vapor pressure | 0.329Pa | | refractive index | 1.3920 (estimate) | | storage temp. | room temp | | solubility | H2O: 0.1 g/mL, clear, colorless | | pka | 13.24±0.20(Predicted) | | form | Crystalline Powder | | color | White | | Odor | at 100.00?%. odorless | | biological source | synthetic | | Water Solubility | SOLUBLE | | Sensitive | Hygroscopic | | Merck | 14,10085 | | BRN | 1720523 | | Dielectric constant | 40.0(Ambient) | | InChIKey | HEBKCHPVOIAQTA-QWWZWVQMSA-N | | LogP | -2.56 at 22℃ | | CAS DataBase Reference | 87-99-0(CAS DataBase Reference) | | NIST Chemistry Reference | Xylitol(87-99-0) | | EPA Substance Registry System | Xylitol (87-99-0) |
| | Xylitol Usage And Synthesis |
| Chemical Properties | White or almost white, crystalline powder or crystals. | | Chemical Properties | Xylitol occurs as a white, granular solid comprising crystalline,
equidimensional particles having a mean diameter of about
0.4–0.6 mm. It is odorless, with a sweet taste that imparts a cooling
sensation. Xylitol is also commercially available in powdered form,
and several granular, directly compressible forms. | | Chemical Properties | The solubility of D-xylitol (D-xylopentan-1.2.3.4.5-pentaol) in water is approximately
1,690 g/L at room temperature. Xylitol is stable under the common processing
conditions of foods.
Xylitol is, depending on the concentration, similarly or slightly sweeter than
sucrose and noncariogenic.
In the European Union, xylitol is approved as E 967 for a large number of food
applications. In the United States, it is approved for use in foods following Good
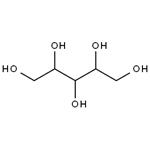
Manufacturing Practice and it is also approved in many other countries. | | History | Xylitol is equally as sweet as sucrose. This property is of advantage to food processors because in reformulating a product from sucrose to xylitol, approximately the same amounts of xylitol can be used. Because xylitol has a negative heat of solution, the substance cools the saliva, producing a perceived sensation of coolness, quite desirable in some food products, notably beverages. Recently, this property has been used in an iced-teaflavored candy distributed in the European market. As of the late 1980s, 28 countries have ruled positively in terms of xylitol for use in commercial products. Xylitol has been found particularly attractive for use in chewing gum, mint and hard candies, and as a coating for pharmaceutical products. Xylitol has the structural formula shown below, with a molecular weight of 152.1. It is a crystalline, white, sweet, odorless powder, soluble in water and slightly soluble in ethanol and methanol. It has no optical activity. | | Uses | Xylitol is a polyhydric alcohol that is a natural sugar substitute com-
mercially made from xylan-containing plants (birch) hydrolyzed to
xylose. it is as sweet as sucrose, dissolves quickly, and has a negative
heat of solution which results in a cooling effect. it has 24 kcal/g.
it is used in chewing gum, throat lozenges, and chocolate. | | Uses | xylitol is a humectant and skin-conditioning agent. It acts as a humidifier, drawing moisture from the air for skin absorption. Some manufacturers also cite a soothing and anti-microbial action. Xylitol is a naturally occurring sugar in birch bark and a range of fibrous fruits and vegetables, including corn. | | Uses | sweetener and excipient, prevents acute otitis media | | Uses | A polyol substrate for xylitol and sorbitol dehydrogenases. | | Uses | As oral and intravenous nutrient; in anticaries preparations. | | Production Methods | Xylitol occurs naturally in many fruits and berries, although
extraction from such sources is not considered to be commercially
viable. Industrially, xylitol is most commonly derived from various
types of hemicellulose obtained from such sources as wood, corn
cobs, cane pulp, seed hulls, and shells. These materials typically
contain 20–35% xylan, which is readily converted to xylose (wood
sugar) by hydrolysis. This xylose is subsequently converted to
xylitol via hydrogenation (reduction). Following the hydrogenation
step, there are a number of separation and purification steps that
ultimately yield high-purity xylitol crystals. The nature of this
process, and the stringent purification procedures employed, result
in a finished product with a very low impurity content. Potential
impurities that may appear in small quantities are mannitol,
sorbitol, galactitol, or arabitol.
Less commonly employed methods of xylitol manufacture
include the conversion of glucose (dextrose) to xylose followed by
hydrogenation to xylitol, and the microbiological conversion of
xylose to xylitol. | | Production Methods | Xylitol is synthesized by reduction of D-xylose catalytically, electrolytically, and by sodium amalgam. D-Xylose is obtained by hydrolysis of xylan [CAS: 9014-63-5] and other hemicellulosic substances obtained from such sources as wood, corn cobs, almond shells, hazelnuts, or olive waste. Isolation of xylose is not necessary; xylitol results from hydrogenation of the solution obtained by acid hydrolysis of cottonseed hulls. Xylitol also is obtained by sodium borohydride reduction of D-xylonic acid γ -lactone and from glucose by a series of transformations through diacetone glucose. | | Definition | ChEBI: A pentitol (five-carbon sugar alcohol) having meso-configuration, being derived from xylose by reduction of the carbonyl group. | | Biotechnological Production | Xylitol is mostly produced by chemical hydrogenation of xylose which is obtained
by hydrolysis of xylans of plants such as birch and beech trees, corn cobs, bagasse,
or straw, but also by fermentation of xylose, for example, using Candida species.
Xylose, especially for hydrogenation, requires a high purity. It may be obtained
from wood extracts or pulp sulfite liquor, a waste product of cellulose production,
by fermentation with a yeast that does not metabolize pentoses. Some strains of
S. cerevisiae, Saccharomyces fragilis, Saccharomyces carlsbergensis, Saccharomyces
pastoanus, and Saccharomyces marxianus are suitable for this purpose.
Hydrolysates of xylan-rich material are often treated with charcoal and ionexchangers
to remove by-products causing problems in hydrogenation or
fermentation.
Many studies of xylitol production by fermentation have been published.
Different organisms, substrates, and conditions were investigated. As the starting
material, xylose or xylose in combination with glucose was used. Fermentation
was carried out in batch reactors as well as continuously.
Among the variations studied was cell recycling in a submerged membrane
bioreactor for C. tropicalis with a high productivity of 12 g/Lh, a conversion rate
of 85 % and a concentration of 180 g/L. Many studies addressed the
immobilization of cells such as S. cerevisiae, C. guilliermondii, or
D. hansenii, especially with calcium alginate. | | General Description | Xylitol is a naturally occurring five carbon sugar alcohol, equivalent to sucrose in sweetness. Xylitol finds applications in the preparation of confectionaries, chewing gum, toothpaste and mouthwashes. Xylitol is a low-energy sweetener with insulin independent metabolism, making it a promising alternative for sugar in diabetic patients. Xylitol is a natural anticaries agent used in the treatment of dental caries, as it is not utilized by cariogenic bacteria creates a starvation effect on them. Xylitol prevents otitis and upper respiratory tract infections. Commercially, microorganisms like bacteria, fungi and yeasts produce xylitol by fermentation. | | Flammability and Explosibility | Non flammable | | Pharmaceutical Applications | Xylitol is used as a noncariogenic sweetening agent in a variety of
pharmaceutical dosage forms, including tablets, syrups, and coatings.
It is also widely used as an alternative to sucrose in foods and
as a base for medicated confectionery. Xylitol is finding increasing
application in chewing gum, mouthrinses, and toothpastes
as an agent that decreases dental plaque and tooth decay (dental
caries). Unlike sucrose, xylitol is not fermented into cariogenic acid
end products and it has been shown to reduce dental caries by
inhibiting the growth of cariogenic Streptococcus mutans bacteria. As xylitol has an equal sweetness intensity to sucrose,
combined with a distinct cooling effect upon dissolution of the
crystal, it is highly effective in enhancing the flavor of tablets and
syrups and masking the unpleasant or bitter flavors associated with
some pharmaceutical actives and excipients.
In topical cosmetic and toiletry applications, xylitol is used
primarily for its humectant and emollient properties, although it has
also been reported to enhance product stability through a
combination of potentiation of preservatives and its own bacteriostatic
and bactericidal properties.
Granulates of xylitol are used as diluents in tablet formulations,
where they can provide chewable tablets with a desirable sweet taste
and cooling sensation, without the ‘chalky’ texture experienced
with some other tablet diluents. Xylitol solutions are employed in
tablet-coating applications at concentrations in excess of 65% w/w.Xylitol coatings are stable and provide a sweet-tasting and durable
hard coating.
In liquid preparations, xylitol is used as a sweetening agent and
vehicle for sugar-free formulations. In syrups, it has a reduced
tendency to ‘cap-lock’ by effectively preventing crystallization
around the closures of bottles. Xylitol also has a lower water
activity and a higher osmotic pressure than sucrose, therefore
enhancing product stability and freshness. In addition, xylitol has
also been demonstrated to exert certain specific bacteriostatic and
bactericidal effects, particularly against common spoilage organisms.
Therapeutically, xylitol is additionally utilized as an energy
source for intravenous infusion therapy following trauma. | | Biochem/physiol Actions | A sugar alcohol sweetener detectable by humans. Produced from hemicellulose hydrolysate fermentation. | | Safety Profile | Very low toxicity by
ingestion. When heated to decomposition it
emits acrid smoke and irritating fumes. A
sugar. | | Safety | Xylitol is used in oral pharmaceutical formulations, confectionery,
and food products, and is generally regarded as an essentially
nontoxic, nonallergenic, and nonirritant material.
Xylitol has an extremely low relative glycemic response and is
metabolized independently of insulin. Following ingestion of
xylitol, the blood glucose and serum insulin responses are
significantly lower than following ingestion of glucose or sucrose.
These factors make xylitol a suitable sweetener for use in diabetic or
carbohydrate-controlled diets.
Up to 100 g of xylitol in divided oral doses may be tolerated
daily, although, as with other polyols, large doses may have a
laxative effect. The laxative threshold depends on a number of
factors, including individual sensitivity, mode of ingestion, daily
diet, and previous adaptation to xylitol. Single doses of 20–30 g and
daily doses of 0.5–1.0 g/kg body-weight are usually well tolerated
by most individuals. Approximately 25–50% of the ingested xylitol
is absorbed, with the remaining 50–75% passing to the lower gut,
where it undergoes indirect metabolism via fermentative degradation
by the intestinal flora.
An acceptable daily intake for xylitol of ‘not specified’ has been
set by the WHO since the levels used in foods do not represent a
hazard to health.
LD50 (mouse, IP): 22.1 g/kg
LD50 (mouse, IV): 12 g/kg
LD50 (mouse, oral): 12.5 g/kg
LD50 (rat, oral): 17.3 g/kg
LD50 (rat, IV): 10.8 g/kg
LD50 (rabbit, oral): 16.5 g/kg
LD50 (rabbit, IV): 4 g/kg | | storage | Xylitol is stable to heat but is marginally hygroscopic. Caramelization
can occur only if it is heated for several minutes near its boiling
point. Crystalline material is stable for at least 3 years if stored at
less than 65% relative humidity and 25℃. Milled and specialized
granulated grades of xylitol have a tendency to cake and should
therefore be used within 9 to 12 months. Aqueous xylitol solutions
have been reported to be stable, even on prolonged heating and
storage. Since xylitol is not utilized by most microorganisms, products made with xylitol are usually safe from fermentation and
microbial spoilage.
Xylitol should be stored in a well-closed container in a cool, dry
place. | | Incompatibilities | Xylitol is incompatible with oxidizing agents. | | Regulatory Status | GRAS listed. Approved for use as a food additive in over 70
countries worldwide, including Europe, the USA and Japan.
Included in the FDA Inactive Ingredients Database (oral solution,
chewing gum). Included in nonparenteral medicines licensed in the
UK and USA. Included in the Canadian List of Acceptable Nonmedicinal
Ingredients. |
| | Xylitol Preparation Products And Raw materials |
|