| Company Name: |
Mainchem Co., Ltd.
|
| Tel: |
+86-0592-6210733 |
| Email: |
sale@mainchem.com |
| Products Intro: |
Product Name:Vinyl ether
CAS:109-93-3
|
- Vinyl ether
-

- $1.00 / 1KG
-
2020-01-13
- CAS:109-93-3
- Min. Order: 1KG
- Purity: 98%-99.9%
- Supply Ability: 100kg
|
| | Vinyl ether Basic information |
| Product Name: | Vinyl ether | | Synonyms: | 1,1'-oxybisethene;divinyl oxide;Vinesthesin;Vinethene;Vinydan;vinyloxyethylene;(vinyloxy)ethene;Vinyl ether | | CAS: | 109-93-3 | | MF: | C4H6O | | MW: | 70.09 | | EINECS: | 203-720-5 | | Product Categories: | | | Mol File: | 109-93-3.mol | 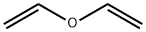 |
| | Vinyl ether Chemical Properties |
| Melting point | -101.1°C | | Boiling point | bp 28.4° | | density | d2020 0.774; d420 0.773 | | refractive index | nD20 1.3989 | | Water Solubility | 5.25g/L(37 ºC) | | Dielectric constant | 3.9(20℃) | | EPA Substance Registry System | Ethene, 1,1'-oxybis- (109-93-3) |
| RIDADR | 1167 | | HazardClass | 3.1 | | PackingGroup | I | | Toxicity | LC50 (3 hr in air) in mice, rats (mg/l): 146.8, 386.5 (Molitor) |
| | Vinyl ether Usage And Synthesis |
| Description | Divinyl ether is a mobile,
colorless, inflammable liquid with a characteristic
sweet odor, bp 28.4 ?C. Approximately 4 %
ethanol is added as a stabilizer. Divinyl ether
may contain 0.0077 % of a stabilizer, phenyl-α-
naphthylamine, that produces a slightly reddish
fluorescence. | | Chemical Properties | Clear, colorless liquid with a characteristicodor; boils at 28.5°C (83°F); density 0.774[at 20°C (68°F)]; soluble in most organicsolvents, slightly soluble in water [0.53 g/dLat 37°C (98°F)]. | | Uses | Copolymer with 3–5% polyvinyl chloride
for plastic products such as clear plastic bottles;
medicine (anesthetic, for brief operations only). | | Uses | Vinyl ether is used as an anesthetic and inorganic synthesis. | | Indications | Divinyl ether is used for minor
surgery, obstetrics, dentistry, and inducing anesthesia.
A certain degree of hepatotoxicity rules
out long-term use. Hypersecretion has also been
observed. Divinyl ether increases and decreases
in tissue levels faster than diethyl ether. | | Definition | ChEBI: Vinyl ether is an ether. | | General Description | A clear colorless liquid with a characteristic odor. Flash point below -22°F. Less dense than water. Autoignition temperature 680°F. Slightly soluble in water; miscible with alcohol, acetone, chloroform, and ether. Must be protected from light.Vapors heavier than air. Toxic by inhalation. | | Air & Water Reactions | Highly flammable. Slightly soluble in water. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. | | Reactivity Profile | Ethers, such as DIVINYL ETHER, can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert. Vinyl ether is miscible with alcohol, acetone, chloroform and ether and must be protected from light [Hawley]. | | Health Hazard | Vinyl ether exhibits low inhalation toxicity.Like ethyl ether, inhalation of its vapors pro duces an anesthetic effect. At concentrationsabove 5% (by volume in air), it can causeunconsciousness in humans. Exposure to a5.1% concentration of its vapor (in air) waslethal to mice.
Divinyl ether exhibited mutagenicity inmicrosomal assay and microbial mutationtests. There is no report on its carcinogenicityin animals or humans. | | Health Hazard | Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | Highly flammable; flash point (closed cup) <
-30°C (<-22°F); vapor density 2.4 (air =
1); the vapor is heavier than air and can travel
a considerable distance to a source of igni tion and flash back; autoignition temperature
360°C (680°F); can be ignited by lightning
or static electricity; fire-extinguishing agent:
dry chemical, foam, or CO2; use a water
spray to keep fire-exposed containers cool
and to disperse the vapors
Vinyl ether forms explosive mixtures with
air, with LEL and UEL values of 1.7%
and 27% by volume of air, respectively. It
forms unstable peroxides on long standing or
in the presence of oxygen. These peroxides
can explode spontaneously or when heated.
It decomposes to acetaldehyde (toxic and
highly flammable) when exposed to light. | | Safety Profile | Mildly toxic by
inhalation. Mutation data reported.
Prolonged exposure causes liver injury. A
very dangerous fire hazard when exposed to
heat or flame; can react vigorously with
oxidizing materials. A severe explosion
hazard in the form of vapor when exposed
to heat or flame. Forms peroxides when
exposed to air or oxygen. Hypergolic
reaction with concentrated nitric acid. To
fight fire, use CO2, dry chemical. When
heated to decomposition it emits acrid
smoke and irritating fumes. Used as an
inhalation anesthetic. See also ETHERS. | | Synthesis | Divinyl ether is prepared by the reaction of
liquefied alkali hydroxide or sodium alkoxide
on β,β_x0002_-dihalodiethyl ether or by pyrolysis
of α,α_x0002_-dichlorodiethyl ether in the gas phase at
600 – 800 ?C in the presence of barium chloride
catalyst . | | storage | Vinyl ether is stored in a flammable-liquidsstorage room or cabinet separated from oxidizing and combustible materials. It shouldbe protected from physical damage andfrom static electricity or lightning. It isshipped in amber glass bottles or metaldrums. | | Waste Disposal | Vinyl ether is mixed with an excess ofhigher-boiling solvent and burned in a chem ical incinerator equipped with an afterburnerand scrubber. Small amounts, free of peroxides, can be evaporated in a fume hood awayfrom a source of ignition. |
| | Vinyl ether Preparation Products And Raw materials |
|