| Company Name: |
Mainchem Co., Ltd.
|
| Tel: |
+86-0592-6210733 |
| Email: |
sale@mainchem.com |
| Products Intro: |
Product Name:Potassium fluoroacetate
CAS:23745-86-0
|
|
| | Potassium fluoroacetate Basic information |
| Product Name: | Potassium fluoroacetate | | Synonyms: | Potassium fluoroacetate;Kaliumfluoracetat;Acetic acid, fluoro-, potassium salt;Fluoroacetic acid potassium salt;Potassium monofluoroacetate | | CAS: | 23745-86-0 | | MF: | C2H2FKO2 | | MW: | 116.1327832 | | EINECS: | | | Product Categories: | | | Mol File: | 23745-86-0.mol | 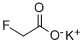 |
| | Potassium fluoroacetate Chemical Properties |
| RIDADR | 2628 | | HazardClass | 6.1(a) | | PackingGroup | I |
| | Potassium fluoroacetate Usage And Synthesis |
| Chemical Properties | Colourless solid | | General Description | A fine, white odorless powder. Toxic by ingestion, inhalation and/or skin absorption. Used as a rodenticide. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | Salts, basic, such as Potassium fluoroacetate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. | | Health Hazard | Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways. | | Safety Profile | Poison by ingestion,
intravenous, parenteral, and subcutaneous
routes. When heated to decomposition it
emits toxic fumes of F and K2O. See also
FLUORIDES. |
| | Potassium fluoroacetate Preparation Products And Raw materials |
|