|
|
| | 4,4'-Bis(2-bromoacetyl)biphenyl Basic information |
| Product Name: | 4,4'-Bis(2-bromoacetyl)biphenyl | | Synonyms: | 1,1'-[1,1'-biphenyl]-4,4'-diylbis[2-bromoethan-1-one];1,1'-(1,1'-Biphenyl-4,4'-diyl)bis(2-bromoethanone);4,4'-Bis(bromoacetyl)biphenyl;Einecs 223-785-3;Ethanone, 1,1'-(1,1'-biphenyl)-4,4'-diylbis(2-bromo-;4,4'-Bis(2-bromoacetyl)biphenyl;2-Bromo-1-[4-[4-(2-bromoacetyl)phenyl]phenyl]ethanone;4,4'-Bis(2-bromoacetyl)biphenyl
1,1'-(1,1'-Biphenyl)-4,4'-diylbis(2-bromoethan-1-one) | | CAS: | 4072-67-7 | | MF: | C16H12Br2O2 | | MW: | 396.07 | | EINECS: | 223-785-3 | | Product Categories: | | | Mol File: | 4072-67-7.mol | 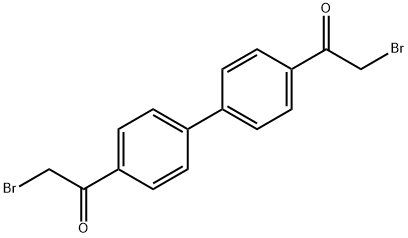 |
| | 4,4'-Bis(2-bromoacetyl)biphenyl Chemical Properties |
| | 4,4'-Bis(2-bromoacetyl)biphenyl Usage And Synthesis |
| Uses | 4,4'-Bis(2-bromoacetyl)biphenyl is an intermediate in laboratory research and development. |
| | 4,4'-Bis(2-bromoacetyl)biphenyl Preparation Products And Raw materials |
|