- 1,2-EPOXYBUTANE
-
- $100.00 / 1KG
-
2023-12-26
- CAS:106-88-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- n-Butylene-1,2-Oxide
-

- $5.00 / 165Kg/Drum
-
2023-01-06
- CAS:106-88-7
- Min. Order: 13200KG
- Purity: 99.5%
- Supply Ability: 1000000000
- 1,2-Epoxybutane 99%
-

- $15.00 / 1KG
-
2021-07-02
- CAS:106-88-7
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | 1,2-EPOXYBUTANE Basic information |
| Product Name: | 1,2-EPOXYBUTANE | | Synonyms: | (R,S)-2-Ethyl-oxirane;1,2-Butene oxide;1,2-buteneoxide;1,2-butyleneepoxide;1,2-butyleneoxide,stabilized;1,2-epoxy;1,2-epoxy-butan;1,2-epoxybutane(1,2-butyleneoxide) | | CAS: | 106-88-7 | | MF: | C4H8O | | MW: | 72.11 | | EINECS: | 203-438-2 | | Product Categories: | Industrial/Fine Chemicals;Oxiranes;Simple 3-Membered Ring Compounds;Organics | | Mol File: | 106-88-7.mol | 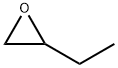 |
| | 1,2-EPOXYBUTANE Chemical Properties |
| Melting point | -129.28°C | | Boiling point | 63 °C(lit.) | | density | 0.829 g/mL at 20 °C(lit.) | | vapor density | 2.2 (vs air) | | vapor pressure | 140 mm Hg ( 20 °C) | | refractive index | n20/D 1.384 | | Fp | 10 °F | | storage temp. | Store below +30°C. | | solubility | 86.8g/l | | form | Colorless liquid with
pungent odor | | color | Colorless to Almost colorless | | PH | 7 (50g/l, H2O, 20℃) | | explosive limit | 1.7-19%(V) | | Water Solubility | 86.8g/L at 25℃ | | BRN | 102411 | | Stability: | Stable, but prone to polymerization - stabilizer may be added to neat liquid. Highly flammable. Incompatible with strong oxidizing agents, acids, bases, anhydrous metal halides, amino, hydroxyl and carboxyl-containing compounds. Inorganic acids and charcoal may lead to polymerization. Heat, light and moisture sensitive. | | InChIKey | RBACIKXCRWGCBB-UHFFFAOYSA-N | | LogP | 0.68 at 25℃ | | CAS DataBase Reference | 106-88-7(CAS DataBase Reference) | | IARC | 2B (Vol. 47, 71) 1999 | | EPA Substance Registry System | 1,2-Butylene oxide (106-88-7) |
| | 1,2-EPOXYBUTANE Usage And Synthesis |
| Description | Butylene oxide is a watery white liquid withan etherial odor. Molecular weight = 72.1 (1,2- and 2,3-isomers);Specific gravity (H2O:1) = 0.83; Boilingpoint = 63.3℃; Freezing/Melting point = - 130℃; Vaporpressure = 142 mmHg at 20℃; 176 mmHg at 25℃;Relative vapor density (air = 1) = 2.2; Relative density ofthe vapor/air mixture at 20�C (air = 1) = 1.3; Flashpoint = - 22℃; Autoignition temperature = 439℃.Explosive limits in air: LEL: 1.7%; UEL: 19%. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 3, Reactivity 2. Soluble in water;solubility = 9.5% at 25℃. | | Chemical Properties | 1,2-Butylene oxide is a colorless mobile liquid with an unpleasant smell. This low boiling liquid has but limited water solubility, yet is miscible with most common organic solvents. It undergoes the usual reactions of epoxides with compounds having labile hydrogen atoms. Some of these are acids, amines, ammonia, alcohols, phenols, polyols, thiols, etc. Butylene oxide can be polymerized or copolymerized with other alkylene oxides to yield polyethers. The resulting polymers are less water soluble than the polymers made from ethylene and propylene oxide, of equivalent chain length. | | Chemical Properties | Butylene oxide is a watery-white liquid with
and ethereal odor. | | Uses | Primarily used as a stabilizer for chlorinated
hydrocarbon solvents; also used as a
chemical intermediate in the production of
butylene glycols | | Uses | Intermediate for various polymers, stabilizer for
chlorinated solvents. | | Definition | ChEBI: 1,2-Epoxybutane is an epoxide. | | General Description | A clear colorless volatile liquid with an ethereal odor. Flash point near 0°F. Density about 6.9 lb / gal. Soluble in water. Boiling point near 140°F. Flammable over a wide range of vapor-air concentrations. May polymerize with the evolution of heat and possible rupture of container if contaminated. Vapors irritate eyes, skin and respiratory system. Prolonged contact with skin may cause in delayed burns. Vapors are heavier than air. Used as an intermediate to make various polymers. Chemicals that polymerize are often stabilized by refrigeration. | | Air & Water Reactions | Highly flammable. Soluble in water and may decompose upon contact with water. | | Reactivity Profile | Epoxides, such as 1,2-EPOXYBUTANE, are highly reactive. They polymerize in the presence of catalysts or when heated. Contact with anhydrous metal halides; amino, hydroxyl and carboxyl functions; inorganic acids and charcoal may cause polymerization. These polymerization reactions can be violent. Compounds in this group react with acids, bases, and oxidizing and reducing agents. They react, possibly violently with water in the presence of acid and other catalysts. | | Hazard | Toxic concentration of vapors occurs at
room temperature. Highly flammable, dangerous
fire risk. Possible carcinogen. | | Health Hazard | Inhalation: intolerable odor and irritation; respiratory injury may occur at higher levels. Ingestion causes irritation of mouth and stomach. Contact with either liquid or vapor may cause burns of eyes. Liquid produces frostbite-type of skin burn if free to evaporate; if confined to skin, burn may cause skin sensitization; not readily absorbed in toxic amounts. | | Fire Hazard | Behavior in Fire: Containers may explode in fire. Use water to cool container from safe distance. | | Flammability and Explosibility | Highly flammable | | Safety Profile | Confirmed carcinogen
with experimental carcinogenic data.
Moderately toxic by ingestion and skin
contact. Mtldly toxic by inhalation.
Experimental reproductive effects. Mutation
data reported. Dangerous fire hazard when
exposed to heat, flame, or powerful
oxidizers. To fight fire, use dry chemical,
water spray, mist or fog, alcohol foam.
When heated to decomposition it emits
acrid smoke and fumes. | | Potential Exposure | It is used as a stabilizer in chlorinated
solvents, and to make other chemicals, such as gasoline
additives. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24�48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor o | | Carcinogenicity | Exposure to 1000 ppm before and during
gestation did not cause any teratogenic effects
in rats; fetal growth and viability were not
affected despite depressed maternal body
weight gain.6 Rabbits exposed at 250 or
1000ppm 7 hours/day during gestational days
0 to 24 had maternal deaths at both exposure
concentrations. No teratogenic effects were
observed, although the pregnancy rate was
reduced in the high-dose group. 1,2-Epoxybutane
is a direct-acting alkylating agent, and it is
genotoxic in a wide range of assays.
Instilled in the eyes of rabbits, 1,2-
epoxybutane caused corneal injury.
A threshold limit value (TLV) has not been
established for 1,2-epoxybutane, although US
manufacturers have recommended a voluntary
time-weighted average-threshold limit value of
40ppm. | | storage | Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical acid you should betrained on its proper handling and storage. Protect againstphysical damage. Store only if inhibited. Outside ordetached storage is preferred. Store in tightly closed containers in a cool, well-ventilated area away from incompatible materials listed above. Metal containers involving thetransfer of this chemical should be grounded and bonded.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. Sources of ignition, such assmoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could createa potential fire or explosion hazard. A regulated, markedarea should be established where this chemical is handled,used, or stored in compliance with OSHA Standard1910.1045. | | Shipping | UN3022 1,2-Butylene oxide, stabilized, Hazard
Class: 3; Labels: 3—Flammable liquid | | Purification Methods | Dry it with CaSO4, and fractionally distil it through a long (126cm) glass helices-packed column. The first fraction contains a water azeotrope. [Beilstein 17 II 17.] | | Incompatibilities | May form explosive mixture with air.
Unless inhibited, can form unstable and explosive peroxides. Before entering confined space where this chemical
may be present, check to make sure that an explosive concentration does not exist. Polymerization will occur in the
presence of acids, strong bases and chlorides of tin, iron and
aluminum. Storage tanks and other equipment should be
absolutely dry and free from air, ammonia, acetylene,
hydrogen sulfide, rust and other contaminants. Incompatible
with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline
materials, strong bases, strong acids, oxoacids, epoxides.
Attacks some plastics. May accumulate static electric
charges that can result in ignition of its vapors. A regulated,
marked area should be established where this chemical is
handled, used, or stored in compliance with OSHA Standard
1910.1045. |
| | 1,2-EPOXYBUTANE Preparation Products And Raw materials |
|