| Company Name: |
ZHIWE CHEMTECH CO LTD
|
| Tel: |
021-20221225 13917446399 |
| Email: |
sales@zhiwe.net |
| Products Intro: |
CAS:7004-98-0
|
| Company Name: |
Shaanxi Dideu Newmaterial Co., Ltd.
|
| Tel: |
029-63373950 15353716720 |
| Email: |
1052@dideu.com |
| Products Intro: |
Product Name:3-Methoxyestra-1,3,5(10)-triene-16.α.,17.α.-diol
CAS:7004-98-0
Purity:98% Package:25kg
|
|
| | EPIMESTROL Basic information |
| Product Name: | EPIMESTROL | | Synonyms: | EPIMESTROL;Stimovul;Org 817;NSC 55975;3-Methoxy-17-epiestriol;3-Methoxy)estra-1,3,5(10)-triene-16alpha,17alpha-diol;Estriol Impurity 11;3-Methoxyestra-1,3,5(10)-triene-16.α.,17.α.-diol | | CAS: | 7004-98-0 | | MF: | C19H26O3 | | MW: | 302.41 | | EINECS: | 230-278-0 | | Product Categories: | | | Mol File: | 7004-98-0.mol | 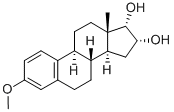 |
| | EPIMESTROL Chemical Properties |
| Melting point | 158-160° | | alpha | D20 +48° (chloroform) | | Boiling point | 450.8±45.0 °C(Predicted) | | density | 1.185±0.06 g/cm3(Predicted) | | pka | 14.53±0.60(Predicted) |
| | EPIMESTROL Usage And Synthesis |
| Originator | Stimovul,Organon,W. Germany,1976 | | Uses | Anterior pituitary activator. | | Definition | ChEBI: Epimestrol is a steroid. It derives from a hydride of an estrane. | | Manufacturing Process | Reduction of 16-keto-17(α)-hydroxyestratrienol-3-methyl to 16,17-
dihydroxyestratrienol-3-methyl ether: A solution of 800 mg of the alpha ketol
methyl ether in 100 cc of ethanol and 10 cc of acetic acid was carefully
maintained at 40°C (water bath), and 200 g of freshly prepared sodium
amalgam (2%) were added in small pieces with efficient swirling. Before all ofthe amalgam had been added, a precipitation of sodium acetate occurred, and
at this point an additional 100 cc of 50% acetic acid were added. After all the
reducing agent had been added, the mixture was transferred to a separatory
funnel with ether and water. The mercury plus aqueous phase was separated,
after partitioning, from the ether; the latter may be further washed with
water, with 0.5N sodium hydroxide, and again with water to purify the alpha
glycol. Evaporation of the ethereal phase yielded a crystalline residue of the
isomeric transoid (16(β),17(α)-dihydroxy-steroid-3-methyl ether and cisoid
16(α),17(α)dihydroxy-steroid-3-methyl ether. | | Therapeutic Function | Anterior pituitary activator |
| | EPIMESTROL Preparation Products And Raw materials |
|