- Butylparaben
-

- $0.00 / 25Kg/Drum
-
2024-04-24
- CAS:94-26-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt/year
- Butylparaben
-

- $6.00 / 1KG
-
2024-04-19
- CAS:94-26-8
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 2000KG/MONTH
- Butylparaben
-

- $0.00 / 1kg
-
2023-12-25
- CAS:94-26-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000000
|
| Product Name: | Butylparaben | | Synonyms: | Butyl 4-Hydroxybenzoate
(Butyl Paraben);Butyl 4-hydroxybenzoate >=99.0% (GC);Butyl 4-hydroxybenzoate Certified Reference Material;4-Hydroxybenzoic acid-n-butyl ester Solution, 100ppm;4-hydroxy-benzoicacibutylester;P-HYDROXYBENZOIC ACID BUTYL ESTER;butylparaaben;BUTYL 4-HYDROXYBENZOATE >=99% | | CAS: | 94-26-8 | | MF: | C11H14O3 | | MW: | 194.23 | | EINECS: | 202-318-7 | | Product Categories: | Aromatics;Pharmaceutical Raw Materials;API;94-26-8 | | Mol File: | 94-26-8.mol | 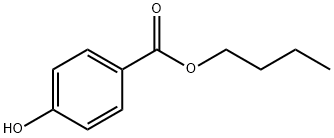 |
| | Butylparaben Chemical Properties |
| Melting point | 67-70 °C(lit.) | | Boiling point | 156-157 °C3.5 mm Hg(lit.) | | density | 1.28 | | vapor pressure | 0.002-0.113Pa at 20-50℃ | | refractive index | 1.5115 (estimate) | | FEMA | 2203 | BUTYL P-HYDROXY BENZOATE | | Fp | 181℃ | | storage temp. | 2-8°C | | solubility | Soluble in DMSO, ethyl acetate, methanol. | | pka | pKa 8.5 (Uncertain) | | form | Crystalline Powder | | color | White to almost white | | Odor | very faint phenolic | | Odor Type | bland | | Water Solubility | <0.1 g/100 mL at 17 ºC | | Merck | 14,1584 | | JECFA Number | 870 | | BRN | 1103741 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong alkalies. | | InChIKey | QFOHBWFCKVYLES-UHFFFAOYSA-N | | LogP | 3.57 | | CAS DataBase Reference | 94-26-8(CAS DataBase Reference) | | NIST Chemistry Reference | P-hydroxybenzoic acid, n-butyl ester(94-26-8) | | EPA Substance Registry System | Butylparaben (94-26-8) |
| | Butylparaben Usage And Synthesis |
| Chemical properties | Butylparaben appears as white crystal powder, having slightly special odor. It is slightly soluble in water, being soluble in alcohol, ether and chloroform. | | Production method | Butylparaben is derived from the esterification between p-hydroxybenzoic acid and butanol. Butanol and p-hydroxybenzoic acid are heated together for being dissolved, slowly added dropwise of sulfuric acid, continue the refluxing for 8h. After cooling, add 4% sodium carbonate solution, separate the water layer, steam out the butanol, let it cool, filter to obtain the crude product, and then carry out ethanol recrystallization (solubility in ethanol: 200g/100ml).
Take sulfuric acid as a catalyst; derive it from the reaction between p-hydroxybenzoic acid and butanol. | | Uses | Butylparaben is used as a preservative in some foods, cosmetics, and drug formulations. It has been added to solutions such as commercially prepared low-ionic strength saline (LISS) solutions and beer to retard microbial growth (Judd et al., 1982; Raducan et al., 1994). Parabens in general are most active against molds and yeasts and, to a lesser extent, bacteria. In comparison to other parabens, butylparaben appears to be the best antifungal agent (HSDB, 2003). | | Toxicity | ADI is subject to postponed decision (FAO/WHO, 2001).
LD50: 16.0 g/kg (mouse, subcutaneous injection).
Mice subjecting to short-term toxicity test have gotten inhibited weight increase. There have been reports regarding to the acute dermatitis for human beings. In the p-hydroxybenzoic acid esters, this product gives the best anti-corrosion effect, but also the largest toxicity. | | Content analysis | 2g (accurate to 0.1mg) was taken and dried in silica gel for 5h before being transferred to the flask. Add 40 mL of 1mol/L of sodium hydroxide, flush flasks with water. Cover the surface of the dish and apply a small fire to boil 1h before cooling. Add 5 drops of bromothymol blue solution (TS-56), titrate the excess sodium hydroxide with 1 mol/L sulfuric acid, and make the color of the solution consistent with the buffer containing the same indicator (pH 6.5). Carry out a blank test at the same time and make the necessary calibration. 1ml/L sodium hydroxide per milliliter corresponds to the 194.2 mg of this product (C11H14O3). | | Usage limit | Japan (1998, calculated on p-hydroxybenzoic acid; the data in parentheses is the amount converted into equivalent amount of this product, g/ kg), soy sauce 0.25 g/L (0.35 g/L), vinegar 0.1 g/L (0.14 g/L); Soft drinks and syrup: 0.1 (0.14); fruit sauce: 0.2 (0.28); fruits and vegetables 0.012 (0.016). | | Hazards & Safety Information | Category :Toxic substances
Toxic classification: poisoning
Acute toxicity: Oral-mouse LD50: 13200 mg/kg; celiac-mouse LD50: 230 mg/kg
Stimulation Data: Skin-Guinea Pig 5%/48 hours Mild
Flammability and Hazardous characteristics: Thermal decomposition; pungent irritation Smoke
Storage and transportation characteristics: Treasury: ventilated, low temperature and dry
Fire extinguishing agent: water, dry powder, foam, carbon dioxide | | Description | Butylparaben is an antimicrobial agent used in pharmaceutical suspensions. It is act by inhibiting DNA, RNA, and enzymes (eg, ATPase and phosphotransferase) synthesis. Butylparaben may be used alone or with other parabens, chiefly methylparaben and/or propylparaben, in medications. It is common in many liquid and solid (gel cap) OTC products such as Tylenol, Drixoral, Maalox, and Mylanta. Unfortunately, butylparaben concentrations were seldom identified for OTC or prescription products. No attempt was made to identify butylparaben-containing dietary supplements. | | Chemical Properties | Butylparaben occurs as colorless crystals or a white, crystalline,
odorless or almost odorless, tasteless powder. | | Uses | preservative in many creams, lotions, ointments and other cosmetics, foods (salad dressings, mayonnaise, spiced sauces,
mustard, frozen dairy products, baked products), pharmaceutical preparations and dentifrices. It is active against molds, fungi and
yeasts, but less active against bacteria. ICU. | | Definition | ChEBI: Butylparaben is an organic molecular entity. It is a Standardized Chemical Allergen. The physiologic effect of butylparaben is by means of Increased Histamine Release, and Cell-mediated Immunity. | | Preparation | Butyl paraben is prepared by esterifying p-hydroxybenzoic acid with butyl alcohol in the presence of an acid catalyst, such
as sulfuric acid, and an excess of the specific alcohol. | | Production Methods | Butylparaben is prepared by esterification of p-hydroxybenzoic acid
with n-butanol. | | General Description | Odorless white crystals or crystalline powder. Tasteless, but numbs the tongue. Aqueous solutions slightly acidic to litmus. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Butylparaben is incompatible with strong oxidizing agents and strong caustics. | | Fire Hazard | Flash point data for Butylparaben are not available; however, Butylparaben is probably combustible. | | Pharmaceutical Applications | Butylparaben is widely used as an antimicrobial preservative in
cosmetics and pharmaceutical formulations.
It may be used either alone or in combination with other paraben
esters or with other antimicrobial agents. In cosmetics, it is the
fourth most frequently used preservative.
As a group, the parabens are effective over a wide pH range and
have a broad spectrum of antimicrobial activity, although they are
most effective against yeasts and molds.
Owing to the poor solubility of the parabens, paraben salts,
particularly the sodium salt, are frequently used in formulations.
However, this may raise the pH of poorly buffered formulations.
See Methylparaben for further information. | | Contact allergens | This substance is one of the parabens family. Parabens are esters formed by p-hydroxybenzoic acid
and an alcohol. They are largely used as biocides in
cosmetics and toiletries, medicaments, or food. They
have synergistic power with other biocides. Parabens
can induce allergic contact dermatitis, mainly in
chronic dermatitis and wounded skin. | | Safety | Butylparaben and other parabens are widely used as antimicrobial
preservatives in cosmetics and oral and topical pharmaceutical
formulations.
Systemically, no adverse reactions to parabens have been
reported, although they have been associated with hypersensitivity
reactions generally appearing as contact dematitis. Immediate
reactions with urticaria and bronchospasm have occurred rarely.
See Methylparaben for further information.
LD50 (mouse, IP): 0.23 g/kg
LD50 (mouse, oral): 13.2 g/kg | | storage | Aqueous butylparaben solutions at pH 3–6 can be sterilized by
autoclaving, without decomposition. At pH 3–6, aqueous
solutions are stable (less than 10% decomposition) for up to about
4 years at room temperature, while solutions at pH 8 or above are
subject to rapid hydrolysis (10% or more after about 60 days at
room temperature). | | Incompatibilities | The antimicrobial activity of butylparaben is considerably reduced
in the presence of nonionic surfactants as a result of micellization.
Absorption of butylparaben by plastics has not been reported but
appears probable given the behavior of other parabens. Some
pigments, e.g. ultramarine blue and yellow iron oxide, absorb
butylparaben and thus reduce its preservative properties.
Butylparaben is discolored in the presence of iron and is subject
to hydrolysis by weak alkalis and strong acids. | | Regulatory Status | Butylparaben is regulated by the U.S. Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA) and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). In 1998 its pesticide registration status was listed as "cancelled" (U.S. EPA, 2003).
Included in the FDA Inactive Ingredients Database (injections; oral capsules, solutions, suspensions, syrups and tablets; rectal, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients. |
| | Butylparaben Preparation Products And Raw materials |
|