|
|
| Product Name: | Aconitic Acid | | Synonyms: | 1-Propene-1,2,3-tricarboxylic acid;3-carboxy-2-pentenedioic acid;3-carboxyglutaconic acid;achilleaic acid;citridinic acid;TRAN-1,2,3-PROPENE TRICARBOXYLIC ACID;ACONITIC ACID;Aconic acid;(1,2,3-Propenetricarboxylic acid) | | CAS: | 499-12-7 | | MF: | C6H6O6 | | MW: | 174.11 | | EINECS: | 2078770 | | Product Categories: | | | Mol File: | 499-12-7.mol | 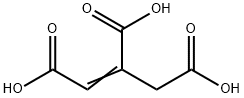 |
| | Aconitic Acid Chemical Properties |
| Melting point | 192°C (rough estimate) | | Boiling point | 225.05°C (rough estimate) | | density | 1.4285 (rough estimate) | | refractive index | 1.5860 (estimate) | | FEMA | 2010 | ACONITIC ACID | | pka | 2.39±0.36(Predicted) | | Odor | Vegetative, musty and nutty with a dry, toasted nuance | | JECFA Number | 627 | | LogP | 0.63 | | EPA Substance Registry System | 1-Propene-1,2,3-tricarboxylic acid (499-12-7) |
| | Aconitic Acid Usage And Synthesis |
| Identification |
CAS.No.:
499-12-7
FL.No.:
8.033
FEMA.No.:
2010
NAS.No.:
2010
CoE.No.:
33
EINECS.No.:
207-877-0
JECFA.No.:
627
| | Description | Aconitic acid has pleasant, winey, acid taste; almost odorless. Aconitic acid of the trans-configuration can be isolated during the processing of sugarcane by precipitating it as the calcium salt from cane syrup or molasses. The concentration in molasses ranges from 1.8 to 2.5%. Aconitic acid may be synthesized from citric acid by dehydration with sulfuric acid or by catalytic dehydration. The cis-configuration is somewhat unstable and is readily rearranged to the trans form by heating. Trans-aconitic acid is decarboxylated to itaconic acid by heating to 180°F. The cis form occurs in plant and animal tissues as a metabolic intermediate in the Krebs cycle during the isomerization of citric acid to isocitric acid by the action of enzyme aconitase.
| | Regulatory Status | CoE: Approved. Bev.: 2 ppm; Food: 15 ppm
FDA: 21 CFR: 184.1007, 582.60
FDA (other): n/a
JECFA: ADI: Acceptable. No safety concern at current levels of intake when used as a flavoring agent (1999).
| | Usage | Reported uses (ppm): (FEMA, 1994)
Alcoholic beverages
15
20
Nonalcoholic beverages
1.64
3.36
| | Natural occurrence | Reported found in beet root, sugarcane, the leaves and tubers of Aconitum napellus, and other natural products (Pelargonium species).
| | Chemical Properties | Aconitic acid has pleasant, winey, acid taste; almost odorless. Aconitic acid of the trans-configuration can be isolated
during the processing of sugarcane by precipitating it as the calcium salt from cane syrup or molasses. The concentration in molasses
ranges from 1.8 to 2.5%. Aconitic acid may be synthesized from citric acid by dehydration with sulfuric acid or by catalytic dehydration. The cis-configuration is somewhat unstable and is readily rearranged to the trans form by heating. Trans-aconitic acid is
decarboxylated to itaconic acid by heating to 180°F. The cis form occurs in plant and animal tissues as a metabolic intermediate in
the Krebs cycle during the isomerization of citric acid to isocitric acid by the action of enzyme aconitase | | Occurrence | Reported found in beet root, sugarcane, the leaves and tubers of Aconitum napellus, and other natural products
(Pelargonium species). | | Uses | Aconitic Acid is a flavoring substance which occurs in the leaves and tubers of aconitum napellus l. And other ranunculaceae. Transaconitic acid can be isolated during sugar cane processing, by precipitation as the calcium salt from cane sugar or molasses. It may be synthesized by sulfuric acid dehydration of citric acid but not by the methanesulfonic acid method. It is used in a maximum level, as served, of 0.003% for baked goods, 0.002% for alcoholic beverages, 0.0015% for frozen dairy products, 0.0035% for soft candy, and 0.0005% or less for all other food categories. | | Definition | ChEBI: A tricarboxylic acid that is prop-1-ene substituted by carboxy groups at positions 1, 2 and 3. | | Preparation | By dehydration of citric acid with concentrated H2SO4: Aconitic acid prepared by this or other methods has the transconfiguration; the cis-isomer is little known. Trans-aconitic acid can be isolated during sugarcane processing by precipitation as the
calcium salt from cane sugar or molasses. | | Taste threshold values | Taste characteristics at 25 ppm: nutty, vegetative, musty and slightly caramellic |
| | Aconitic Acid Preparation Products And Raw materials |
|