|
|
| | bis(methoxycarbonyl)methylidene-imino-azanium Basic information |
| Product Name: | bis(methoxycarbonyl)methylidene-imino-azanium | | Synonyms: | bis(methoxycarbonyl)methylidene-imino-azanium;2-Diazomalonic acid dimethyl;2-Diazopropanedioic acid dimethyl ester;Diazomalonic acid dimethyl;Diazopropanedioic acid dimethyl ester;Dimethyl Diazomalonate;Diazomalonic acid methyl ester;Malonic acid, diazo-, dimethyl ester | | CAS: | 6773-29-1 | | MF: | C5H7N2O4+ | | MW: | 158.11 | | EINECS: | | | Product Categories: | | | Mol File: | 6773-29-1.mol | 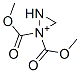 |
| | bis(methoxycarbonyl)methylidene-imino-azanium Chemical Properties |
| Boiling point | 50-52 °C(Press: 1 Torr) | | solubility | soluble in ether, THF, halocarbon and hydrocarbon solvents. |
| | bis(methoxycarbonyl)methylidene-imino-azanium Usage And Synthesis |
| Synthesis Reference(s) | Synthetic Communications, 17, p. 1709, 1987 DOI: 10.1080/00397918708063988
Synthesis, p. 658, 1971 DOI: 10.1055/s-1971-21790 | | Synthesis | The Preparative Method of Dimethyl Diazomalonate: diazomalonates are generally prepared by diazo transfer reaction of malonates and sulfonyl azides. A recent modification employs polystyrene-supported trialkylammonium azide (generated in situ) under phase-transfer catalysis conditions and allows for facile isolation.
| | Precautions | Dimethyl diazomalonate has been utilized more extensively than has diethyl diazomalonate due to the explosion hazard of the latter. Nonetheless, care should be taken in the preparation and handling of dimethyl diazomalonate. Storage at low temperatures is recommended. The decomposition of dimethyl diazomalonate evolves nitrogen and can result in high pressures.
|
| | bis(methoxycarbonyl)methylidene-imino-azanium Preparation Products And Raw materials |
|