- POTASSIUM SULFIDE
-

- $1.00 / 1KG
-
2020-01-10
- CAS:1312-73-8
- Min. Order: 1KG
- Purity: 98% HPLC
- Supply Ability: 10 tons/month
|
| | POTASSIUM SULFIDE Basic information |
| Product Name: | POTASSIUM SULFIDE | | Synonyms: | Potassiumsulphide;Potassium-sulphide-;Potassium sulfide, anhydrous, min. 95%;dipotassium bisulfide;dipotassium sulfanide;"Potassium sulfide, tech.";POTASSIUM SULFIDE;dipotassiummonosulfide | | CAS: | 1312-73-8 | | MF: | K2S | | MW: | 110.26 | | EINECS: | 215-197-0 | | Product Categories: | metal chalcogenides;Inorganics | | Mol File: | 1312-73-8.mol | 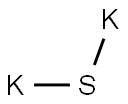 |
| | POTASSIUM SULFIDE Chemical Properties |
| Melting point | 912° | | Boiling point | 912 °C | | density | 1.74 | | solubility | soluble in H2O, ethanol; insoluble in ethyl ether | | form | Powder | | color | white to yellow | | Water Solubility | very soluble H2O, alcohol, glycerol; insoluble ether [MER06] | | Sensitive | air sensitive, hygroscopic | | Stability: | Stable, but air-sensitive. Flammable. Contact with acids liberates poisonous hydrogen sulfide. Anhydrous material may be spontaneously combustible. | | CAS DataBase Reference | 1312-73-8(CAS DataBase Reference) | | EPA Substance Registry System | Potassium sulfide (K2S) (1312-73-8) |
| | POTASSIUM SULFIDE Usage And Synthesis |
| Chemical Properties | colourless solid; turns brownish-red on | | Chemical Properties | Potassium sulfide is a brownish-red crystalline
solid. | | Uses | Potassium Sulfide is reported to be used as a reactant for the efficient synthesis of variously substituted thiophenes through copper-catalyzed tandem S-alkenylation with 1,4-diiodo-1,3-dienes. It is also used as a reactant for the synthesis of KBi6.33S10 and K2Bi8S13 at high temperature. | | Uses | Reagent in analytical chemistry, depilatory,
medicine. | | Production Methods | Potassium sulfide, K2S, yellowish to reddish solid, soluble, formed by heating potassium sulfate and carbon to a high temperature; potassium hydrogen sulfide, potassium bisulfide, potassium acid sulfide KHS, formed in solution by reaction of potassium hydroxide or carbonate solution and excess H2S. | | General Description | A red crystalline solid. Denser than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion. | | Air & Water Reactions | May spontaneously ignite with exposure to air. Deliquescent. Water soluble | | Reactivity Profile | A reducing agent. So readily oxidized as to be pyrophoric in air [Bretherick 1979 p. 120]. POTASSIUM SULFIDE is incompatible with chloroform and nitrogen oxide. | | Hazard | Flammable, dangerous fire risk, may ignite
spontaneously, explosive in the form of dust or pow-
der. | | Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. | | Safety Profile | Poison by ingestion and
inhalation. Emits H2S in contact with acids;
steam. A flammable solid. Unstable; may
explode on percussion or rapid heating.
Ignites on contact with nitrogen oxide.
Reacts with H2O to form KOH and KSH.
When heated to decomposition it emits very toxic fumes of K2O and SOx. See also
SULFIDES. | | Potential Exposure | Potassium sulfide is used as a reagent
in analytical chemistry; and in pharmaceutical preparations. | | Shipping | UN1382 Potassium sulfide, anhydrous or
Potassium sulfide with <30% water of crystallization,
Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible
material. | | Incompatibilities | May explosively decompose from shock,
friction, or concussion. Dust or granules may spontaneously
ignite on contact with air. The aqueous solution is a strong
base; reacts violently with strong acids and acid fumes. The
solid material decomposes on contact with acids producing
hydrogen sulfide, and oxidizers producing sulfur dioxide. |
| | POTASSIUM SULFIDE Preparation Products And Raw materials |
|