Esmolol manufacturers
- Esmolol
-

- $1.00 / 1g
-
2021-01-06
- CAS:81147-92-4
- Min. Order: 1g
- Purity: 85.0-99.8%
- Supply Ability: 20ton
|
| | Esmolol Basic information |
| Product Name: | Esmolol | | Synonyms: | ESMOLOL;4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzenepropanoic acid methyl ester;4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-benzenepropanoicacimethyle;asl8052-001;methyl4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)benzenepropanoate;Methyl 3-[4-(2-hydroxy-3-propan-2-ylamino-propoxy)phenyl]propanoate;Methyl 4-[2-hydoxy-3[(1-methylethyl)amino]-propoxy]benzenepropanoate;3-[4-[2-Hydroxy-3-(isopropylamino)propoxy]phenyl]propanoic acid methyl ester | | CAS: | 81147-92-4 | | MF: | C16H25NO4 | | MW: | 295.37 | | EINECS: | | | Product Categories: | 81147-92-4 | | Mol File: | 81147-92-4.mol | 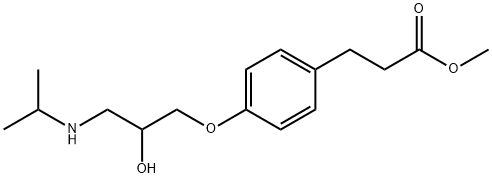 |
| | Esmolol Chemical Properties |
| Melting point | 48-50 °C | | Boiling point | 430.2±40.0 °C(Predicted) | | density | 1.026 | | storage temp. | Store at -20°C | | solubility | DMSO: 59 mg/mL (199.75 mM);Ethanol: 59 mg/mL (199.75 mM) | | pka | 13.88±0.20(Predicted) | | Water Solubility | Slightly soluble | | Merck | 14,3700 | | CAS DataBase Reference | 81147-92-4(CAS DataBase Reference) |
| | Esmolol Usage And Synthesis |
| Originator | Esmolol,AroKor Holdings | | Uses | Agricultural chemical. | | Uses | Esmolol is a cardioselective beta1 receptor blocking agent. | | Definition | ChEBI: Methyl 3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoate is a methyl ester that is methyl 3-(4-hydroxyphenyl)propanoate in which the hydrogen attached to the phenolic hydroxy group is substituted by a 2-hydroxy-3-(isopropylamino)propyl group. It is an aromatic ether, a member of ethanolamines, a methyl ester, a secondary alcohol and a secondary amino compound. It is functionally related to a 3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoic acid. | | Manufacturing Process | A solution of 17 g (0.1 mole) of 3-(4-hydroxyphenyl)propionic acid in 500 mL
methanol and 2 mL concentrated sulfuric acid were placed in a Soxhlet
extractor charged with 3A molecular sieves. The solution was refluxed for 72
hours and the sieve were exchanged at 24 hour intervals. The reaction
medium was then evaporated to an oil which was dissolved in 100 mL toluene
and extracted with 100 mL water (3 times). The toluene phase was dried over
magnesium sulfate, treated with activated charcoal and evaporated to provide
15 g (80%) of a clear oil. The NMR spectrum was consistent with the methyl
3-(4-hydroxyphenyl)propionate.
The oil described above was utilized directly in the condensation reaction with
the epichlorohydrin. A mixture of 0.1 mole of methyl 3-(4-
hydroxyphenyl)propionate, 0.2 mole potassium carbonate and 0.4 mole
epichlorohydrin in 250 mL acetone was heated to reflux for 24 hours. The
reaction medium was then filtered and evaporated. The residue was taken up
in 100 mL toluene and washed with 100 mL 1.0 N NaOH and 100 mL water (2
times). The toluene phase was then dried over magnesium sulfate and
evaporated to provide the crude product as an oil. Purification was effected by
vacuum distillation (156°C/0.4 mm) and provided methyl 3-[4-(2,3-
epoxypropoxy)phenyl]propionate. The NMR and IR spectra and elemental
analysis data were consistent with the assigned structure.
A mixture of 50 g (0.21 mole) of methyl 3-[4-(2,3-
epoxypropoxy)phenyl]propionate and 100 mL of isopropylamine in 100 mLmethanol was heated to reflux for 4 hours. The reaction medium was then
evaporated and the resulting oil taken up in methanol and treated with
ethereal HCl and provided crystals which were recrystallized in similar fashion
to provide 28 g (47%) of white crystals: melting point 85-86°C. The NMR and
IR spectra and the elemental analysis data were consistent with the structure
of methyl 4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)benzenepropanoate.
In practice it is usually used as hydrochloride. | | Therapeutic Function | Beta-adrenergic blocker | | Hazard | A reproductive hazard. | | Clinical Use | Esmolol (Brevibloc) is a short-acting intravenously administered
β1-selective adrenoceptor blocking agent. It
does not possess membrane-stabilizing activity or sympathomimetic
activity.
Esmolol is used in the treatment of supraventricular
tachyarrhythmias for rapid control of ventricular rate
and reduction of myocardial oxygen consumption.
Discontinuation of administration is followed by a rapid
reversal of its pharmacological effects because of esmolol’s
rapid hydrolysis by plasma esterases. | | Side effects | The most frequently reported adverse effects are hypotension,
nausea, dizziness, headache, and dyspnea.As
with many β-blocking drugs, esmolol is contraindicated
in patients with overt heart failure and those in cardiogenic
shock. | | Metabolism | The -blocker esmolol (Brevibloc) is unusual in that
it is very rapidly metabolized; its plasma half-life is only
9 minutes. It is subject to hydrolysis by cytosolic esterases
in red blood cells to yield methanol and an acid
metabolite, the latter having an elimination half-life of
about 4 hours. Only 2% of the administered esmolol is
excreted unchanged. Because of its rapid onset and short duration of action, esmolol is used by the intravenous
route for the control of ventricular arrhythmias
in emergencies. |
| | Esmolol Preparation Products And Raw materials |
|