|
|
| | TAS6417 Basic information |
| Product Name: | TAS6417 | | Synonyms: | TAS6417;TAS-6417;TAS 6417;TAS6417;(S)-N-(4-amino-6-methyl-5-(quinolin-3-yl)-8,9-dihydropyrimido[5,4-b]indolizin-8-yl)acrylamide;2-Propenamide, N-[(8S)-4-amino-8,9-dihydro-6-methyl-5-(3-quinolinyl)pyrimido[5,4-b]indolizin-8-yl]-;CLN-081;TAS6417 TAS-6417;Propenamide, N-[(8S)-4-amino-8,9-dihydro-6-methyl-5-(3-quinolinyl)pyrimido[5,4-b]indolizin-8-yl]- (ACI);Zipalertinib (TAS-6417) | | CAS: | 1661854-97-2 | | MF: | C23H20N6O | | MW: | 396.44 | | EINECS: | | | Product Categories: | APIs;API | | Mol File: | 1661854-97-2.mol | 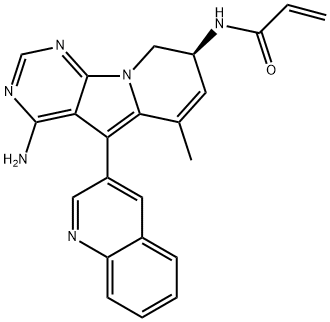 |
| | TAS6417 Chemical Properties |
| Boiling point | 756.6±60.0 °C(Predicted) | | density | 1.40±0.1 g/cm3(Predicted) | | storage temp. | 4°C, protect from light, stored under nitrogen | | solubility | DMSO:20.0(Max Conc. mg/mL);50.44(Max Conc. mM) | | form | Solid | | pka | 12.81±0.40(Predicted) | | color | White to yellow |
| | TAS6417 Usage And Synthesis |
| Uses | Zipalertinib (TAS6417; CLN-081) is a highly effective, orally active and pan-mutation-selective EGFR tyrosine kinase inhibitor with a unique scaffold fitting into the ATP-binding site of the EGFR hinge region, with IC50 values ranging from 1.1-8.0 nM[1][2]. | | in vivo | Zipalertinib (TAS6417) (10-200 mg/kg) causes persistent tumor regression in vivo in EGFR exon 20 insertion-driven tumor models. TAS6417 inhibits mutant EGFR in tumors but not WT EGFR in skin tissues[1].
?
Zipalertinib (TAS6417) had no effect on EGFR-independent proliferation in NCI-H23 or NCI-H460 cells[1].
?
Zipalertinib (TAS6417) administered at 20 mg/kg, which achieves complete suppression of tumor growth, induces a significant decrease in pEGFR, leading to reduction of pAKT and pERK at 1 h. The inhibitory effect is still noted at 6 h, and phosphorylation of EGFR, ATK, and ERK recovered by 24 h[1].
?
Zipalertinib (TAS6417) (100 and 200 mg/kg/day) prolongs survival of animals bearing lung cancer[1].
| Animal Model: | Mice implanted with NCI-H1975 EGFR D770_N771insSVD xenografts[1]. | | Dosage: | 50 and 100 mg/kg. | | Administration: | Orally once daily for 14 days. | | Result: | Showed marked tumor growth inhibition with treatment/control (T/C) ratios of 51% and 19%, respectively. |
| | IC 50 | EGFR: 1.1-8.0 nM (IC50) | | References | [1] Hasako S, et al. TAS6417, A Novel EGFR Inhibitor Targeting Exon 20 Insertion Mutations. Mol Cancer Ther. 2018 Aug;17(8):1648-1658. DOI:10.1158/1535-7163.MCT-17-1206
[2] Hibiki Udagawa, et al. TAS6417/CLN-081 Is a Pan-Mutation-Selective EGFR Tyrosine Kinase Inhibitor with a Broad Spectrum of Preclinical Activity against Clinically Relevant EGFR Mutations. Mol Cancer Res. 2019 Nov;17(11):2233-2243. DOI:10.1158/1541-7786.MCR-19-0419 |
| | TAS6417 Preparation Products And Raw materials |
|