| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:8-Azanebularine
CAS:38874-46-3
Package:50mg/RMB 12900;100mg/RMB 17500;25mg/RMB 9930
|
8-Azanebularine manufacturers
- 8-Azanebularine
-
- $1230.00 / 50mg
-
2025-08-21
- CAS:38874-46-3
- Min. Order:
- Purity:
- Supply Ability: 10g
|
| | 8-Azanebularine Basic information |
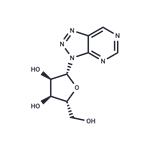
| Product Name: | 8-Azanebularine | | Synonyms: | 8-Azanebularine;3H-1,2,3-Triazolo[4,5-d]pyrimidine, 3-β-D-ribofuranosyl-;(2R,3S,4R,5R)-2-(hydroxymethyl)-5-(triazolo[4,5-d]pyrimidin-3-yl)tetrahydrofuran-3,4-diol | | CAS: | 38874-46-3 | | MF: | C9H11N5O4 | | MW: | 253.22 | | EINECS: | | | Product Categories: | | | Mol File: | 38874-46-3.mol |  |
| | 8-Azanebularine Chemical Properties |
| Boiling point | 615.0±55.0 °C(Predicted) | | density | 2.11±0.1 g/cm3(Predicted) | | pka | 12.80±0.70(Predicted) | | form | Solid | | color | White to yellow |
| | 8-Azanebularine Usage And Synthesis |
| Uses | 8-Azanebularine, a compound with hydrogen in place of the C6 amino group, inhibits the ADAR2 reaction at high concentrations (IC50=15 mM). 8-Azanebularine is incorporated into an RNA structure recognized by human ADAR2 results in high-affinity binding (KD=2 nM). 8-Azanebularine can be used for the research of ADAR-catalyzed RNA-editing reaction[1]. | | IC 50 | ADAR2 reaction: 15 mM (IC50); ADAR2: 2 nM (Kd) | | References | [1] Haudenschild BL, et al. A transition state analogue for an RNA-editing reaction. J Am Chem Soc. 2004;126(36):11213-11219. DOI:10.1021/ja0472073
[2] Mendoza HG, et al. Selective Inhibition of ADAR1 Using 8-Azanebularine-Modified RNA Duplexes. Biochemistry. 2023 Apr 18;62(8):1376-1387. DOI:10.1021/acs.biochem.2c00686 |
| | 8-Azanebularine Preparation Products And Raw materials |
|