|
|
| | 4-(TERT-BUTOXYCARBONYL)BENZOIC ACID Basic information |
| Product Name: | 4-(TERT-BUTOXYCARBONYL)BENZOIC ACID | | Synonyms: | tert-Butyl hydrogen terephthalate;tert-Butyl terephthalate;4-[(2-methylpropan-2-yl)oxycarbonyl]benzoic Acid;mono-(tert-Butyl) terephthalate;4-(TERT-BUTOXYCARBONYL)BENZOIC ACID;TEREPHALIC ACID MONO TERT-BUTYL ESTER;4-(tert-Butoxycarbonyl)benzoic acid, tert-Butyl 4-carboxybenzoate;4-[(2-methylpropan-2-yl)oxy-oxomethyl]benzoic acid | | CAS: | 20576-82-3 | | MF: | C12H14O4 | | MW: | 222.24 | | EINECS: | | | Product Categories: | | | Mol File: | 20576-82-3.mol | 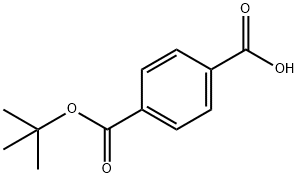 |
| | 4-(TERT-BUTOXYCARBONYL)BENZOIC ACID Chemical Properties |
| Melting point | 209-212(dec.) | | Boiling point | 351.8±25.0 °C(Predicted) | | density | 1.173±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO (Slightly), Ethyl Acetate (Slightly). Methanol (Slightly) | | pka | 3.76±0.10(Predicted) | | form | Solid | | color | White to Off-White |
| Hazard Codes | Xi | | Hazard Note | Irritant | | HS Code | 2918300090 |
| | 4-(TERT-BUTOXYCARBONYL)BENZOIC ACID Usage And Synthesis |
| Uses | Mono-tert-Butyl Terephthalate, is used in the synthesis of pyrrolidinone analogs acting as potential 20S proteasome inhibitors. | | Synthesis | The general procedure for the synthesis of 4-(tert-butoxycarbonyl)benzoic acid from di-tert-butyl terephthalate was as follows: 6.1 g (0.022 mol) of di-tert-butyl terephthalate was suspended in 30 mL of tert-butanol, and 22 mL (0.022 mol) of 1N KOH solution was slowly added. The reaction mixture was heated to 60°C and maintained at this temperature for 7-8 hours. After completion of the reaction, the mixture was cooled, diluted with water and extracted three times with ethyl acetate. After combining the organic phases, the aqueous phase was acidified to acidity with dilute hydrochloric acid and the product was again extracted with ethyl acetate. All ethyl acetate extracts were combined, washed with saturated aqueous sodium chloride solution and dried over anhydrous magnesium sulfate. The desiccant was removed by filtration, and the filtrate was concentrated under reduced pressure to give 4-(tert-butoxycarbonyl)benzoic acid (mono-tert-butyl terephthalate) as a white solid with a yield of 4.7 g, 96% yield, and a melting point of 100-102°C. The product was extracted with dilute hydrochloric acid. | | References | [1] Journal of Medicinal Chemistry, 1992, vol. 35, # 4, p. 641 - 662
[2] Patent: US5221665, 1993, A
[3] Patent: US5232928, 1993, A
[4] Patent: EP369391, 1991, A3
[5] Patent: EP369391, 1990, A2 |
| | 4-(TERT-BUTOXYCARBONYL)BENZOIC ACID Preparation Products And Raw materials |
|