| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Valdecoxib Impurity N
CAS:1709956-95-5
Remarks:An impurity of Valdecoxib. Valdecoxib is a non-steroidal anti-inflammatory drug (NSAID) used for the
|
| Company Name: |
S.Z. PhyStandard Bio-Tech. Co., Ltd.
|
| Tel: |
0755-4000505016 13380397412 |
| Email: |
3001272453@qq.com |
| Products Intro: |
Product Name:Parecoxib
CAS:1709956-95-5
Purity:99% HPLC Package:10mg;25mg;50mg;100mg
|
|
| | Parecoxib Basic information |
| Product Name: | Parecoxib | | Synonyms: | Parecoxib;Valdecoxib Impurity N;Parecoxib Impurity 33;3-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonyl Chloride;Benzenesulfonyl chloride, 3-(5-methyl-3-phenyl-4-isoxazolyl)-;ValdecoxibImpurity39 | | CAS: | 1709956-95-5 | | MF: | C16H12ClNO3S | | MW: | 333.79 | | EINECS: | | | Product Categories: | | | Mol File: | 1709956-95-5.mol | 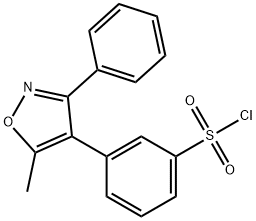 |
| | Parecoxib Chemical Properties |
| Melting point | 165-167°C | | Boiling point | 459.4±45.0 °C(Predicted) | | density | 1.340±0.06 g/cm3(Predicted) | | storage temp. | Refrigerator | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | -3.44±0.50(Predicted) | | form | Solid | | color | White to Off-White |
| | Parecoxib Usage And Synthesis |
| Uses | Anti-inflammatory; analgesic (cyclooxygenase COX-2 inhibitor). | | Uses | 3-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonyl Chloride is an Impurity of Parecoxib Sodium (P193275), an anti-inflammatory, analgesic. | | Clinical Use | Parecoxib is a pro-drug of valdecoxib administered IM or IV for perioperative analgesic and
anti-inflammatory use. As a pro-drug, it undergoes rapid in vivo hydrolysis to valdecoxib.Parecoxib at greater than 20 mg has analgesic activity superior to that of placebo and similar to
that of parenteral 30 or 60 mg of ketorolac in patients with postoperative dental pain. A significant adverse effect is
drug hypersensitivity. Parecoxib is currently marketed worldwide but has not been approved for use in the United
States. | | Synthesis | The acylation of 4-(5-methyl-
3-phenylisoxazol-4-yl)benzenesulfonamide
(valdecoxib), with propionic anhydride in a solution
of triethanolamine (TEA) and 4-dimethylaminophenol
(DMAP) in tetrahydrofuran
gives N-propionamide, which is
treated with NaOH in ethanol to give the sodium
salt of parecoxib . | | Drug interactions | Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possible increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparin, dabigatran and edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlafaxine.
Antidiabetics: possibly enhanced effect of
sulphonylureas.
Antiepileptics: possibly enhanced effect of phenytoin.
Antifungals: if used with fluconazole reduce the dose
of parecoxib.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: potential for increased risk of
nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate,
(possible increased risk of toxicity); increased risk of
bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity; possible
antagonism of diuretic effect; increased risk of
hyperkalaemia with potassium-sparing diuretics.
Lithium: reduced excretion of lithium (risk of
toxicity).
Pentoxifylline: possibly increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity. | | Metabolism | Parecoxib is rapidly and almost completely converted to
valdecoxib and propionic acid.
Elimination of valdecoxib
is by extensive hepatic metabolism involving multiple
pathways, including cytochrome P 450 (CYP) 3A4 and
CYP2C9 isoenzymes and glucuronidation (about 20%) of
the sulphonamide moiety.
Excretion is mainly via the urine with about 70% of a dose
appearing as inactive metabolites. No unchanged parecoxib is
found in the urine with only trace amounts in the faeces. |
| | Parecoxib Preparation Products And Raw materials |
|