- Ticarcillin USP/EP/BP
-
- $1.10 / 1g
-
2021-06-25
- CAS:34787-01-4
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
- Ticarcillin DisodiuM
-

- $1.00 / 1KG
-
2020-05-10
- CAS: 34787-01-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
|
| Product Name: | Ticarcillin | | Synonyms: | (2s,5r,6r)-6-[[(2r)-2-carboxy-2-thiophen-3-yl-acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;TRIARCILLIN;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(carboxy-3-thienylacetyl)amino]-3,3-dimethyl-7-oxo-, [2S-[2a,5a,6b(S*)]]-;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-carboxy-3-thienylacetyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)- (9CI);6-[D-(-)-a-Carboxy-3-thienylacetamido]penicillanic acid;Ticarcillin acid;Ticarcillin Supplement,Ticarcillin;TICARCILLIN | | CAS: | 34787-01-4 | | MF: | C15H16N2O6S2 | | MW: | 384.43 | | EINECS: | 252-213-5 | | Product Categories: | | | Mol File: | 34787-01-4.mol | 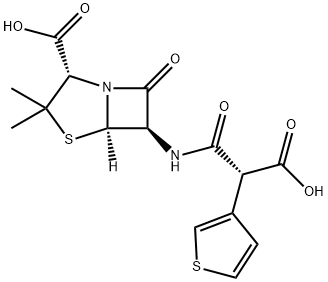 |
| | Ticarcillin Chemical Properties |
| Boiling point | 768.3±60.0 °C(Predicted) | | density | 1.62±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | pKa 2.89±0.05(H2O
t=25.0
I=0.15 (KCl)) (Uncertain);3.28±0.04 (Uncertain) | | CAS DataBase Reference | 34787-01-4(CAS DataBase Reference) |
| | Ticarcillin Usage And Synthesis |
| Brand Name(s) in US | Timentin
| | Description | Temocillin disodium is a broad-spectrum, β-lactamase resistant, injectable penicillin.
High serum levels and a five hour half-life allow once or twice-daily dosing. | | Originator | Beecham (United Kingdom) | | Uses | Ticarcillin is a carboxypenicillin belonging to the beta-lactam class of antibiotics. Ticarcillin is an injectable antibiotic used in the treatment of infections caused by gram-negative bacteria, particularly Pseudomonas aeruginosa. | | Uses | Ticarcillin (SB) is a significant penicillin antibiotic that incorporates the thiophene ring system. | | Uses | Ticarcillin is a carboxypenicillin belonging to the beta-lactam class of antibiotics. Ticarcillin is an injectable antibiotic used in the treatment of infections caused by gram-negative bacteria, part
icularly Pseudomonas aeruginosa. | | Definition | ChEBI: A penicillin compound having a 6beta-[(2R)-2-carboxy-2-thiophen-3-ylacetyl]amino side-group. | | Brand name | TEMOPEN | | Antimicrobial activity | Because it is hydrolyzed less rapidly than ampicillin, non-β-
lactamase-producing strains of N. gonorrhoeae, ampicillin-susceptible
H. influenzae and some Enterobacteriaceae are susceptible.
Most aerobic and anaerobic Gram-positive bacteria are susceptible,
with the exception of E. faecalis and β-lactamase-producing
Staph. aureus. Anaerobic Gram-negative bacteria including B. fragilis
are usually susceptible. Bactericidal synergy with aminoglycosides
is demonstrable against Ps. aeruginosa and enterobacteria. | | Acquired resistance | Ticarcillin is generally cross-resistant with carbenicillin.
It is somewhat stable to hydrolysis by AmpC-mediated β-lactamases of Gram-negative bacilli, but can be hydrolyzed
by most other chromosomally and plasmid-mediated enzymes
unless protected by a β-lactamase inhibitor. | | Pharmacokinetics | Oral absorption: Negligible
Cmax 1 g intramuscular: 35 mg/L after 1 h
Plasma half-life: 1.3 h
Volume of distribution: 0.21 L/kg
Plasma protein binding: 50–60%
Absorption and distribution
It is not orally absorbed. On parenteral co-administration
with gentamicin, the plasma concentration of ticarcillin is
unaffected, but the concentration of gentamicin is lowered. It
enters the serous fluids, providing concentrations up to 60%
of those of the plasma. It does not cross the normal meninges
but levels of up to 50% of those of the plasma can be found
in meningitis.
Metabolism and excretion
Up to 15% is excreted as penicilloic acid, a higher percentage
than for carbenicillin (up to 5%). Some is excreted in
the bile, producing levels 2–3 times those in the plasma, but
the main route of excretion is through the kidneys (80%),
principally as unchanged drug, appearing in the urine in the
first 6 h. It is more rapidly eliminated in children with cystic
fibrosis. | | Clinical Use | Serious infection, including septicemia, respiratory tract infections,
genitourinary tract infections and skin and soft-tissue infections caused
by susceptible bacteria | | Side effects | As with all penicillins, hypersensitivity reactions may occur,
but are less frequent and severe than those associated with
benzylpenicillin. Rashes and eosinophilia occur; reversible
abnormalities of liver function can develop. Since large doses
of the drug have to be used, convulsions can occur, as with
other penicillins, and being a disodium salt, electrolyte disturbances
can result from the sodium load and from loss of
potassium. | | Synthesis | Ticarcillin, [2S-(2|á,5|á,6|?)]-3,3-dimethyl-7-oxo-6-[2-carboxy-2-(3-thienyl)
acetamido]-4-thia-1-azabicyclo[3.2.0]-heptan-2-carboxylic acid (32.1.1.34), is synthesized by direct acylation of 6-APA in the presence of sodium hydroxide, but with
3-thienylmalonic acid chloride (32.1.1.33), which gives ticarcillin. 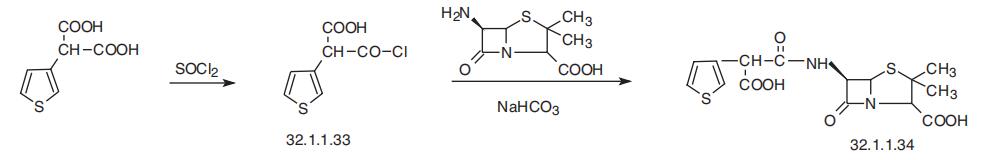
|
| | Ticarcillin Preparation Products And Raw materials |
|