- 2-Aminobenzaldehyde
-

- $0.00 / 25KG
-
2023-10-21
- CAS:529-23-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- 2-Aminobenzaldehyde
-

- $0.00 / 1KG
-
2023-09-06
- CAS:529-23-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- 2-Aminobenzaldehyde
-

- $0.00 / 1g
-
2021-07-20
- CAS:529-23-7
- Min. Order: 10mg
- Purity: 99%HPLC
- Supply Ability: 2000tons
|
| Product Name: | 2-Aminobenzaldehyde | | Synonyms: | Benzaldehyde,2-amino-;2-Aminobenzaldeyhde;o-Amino Benzaldehyde 2-Amino Benzaldehyde;ORTHO-AMINOBENZALDEHYDE;2-AMINOBENZALDEHYDE;2-AMinobenzaldehyde >=98%;2-Formylaniline;Bromhexine Impurity 11 | | CAS: | 529-23-7 | | MF: | C7H7NO | | MW: | 121.14 | | EINECS: | 208-454-3 | | Product Categories: | Carbonyl Compounds;Aldehydes;C7 | | Mol File: | 529-23-7.mol | 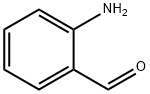 |
| | 2-Aminobenzaldehyde Chemical Properties |
| Melting point | 38°C | | Boiling point | 225.84°C (rough estimate) | | density | 1.1344 (rough estimate) | | refractive index | 1.5323 (estimate) | | Fp | 113 °C | | storage temp. | -20°C | | form | Low Melting Solid | | pka | -0.01±0.10(Predicted) | | color | White to yellow | | Stability: | Unstable: reported to polymerize rapidly at room temperature. Incompatible with strong oxidizing agents, strong bases. Store at -20 C. | | LogP | 1.080 (est) | | CAS DataBase Reference | 529-23-7(CAS DataBase Reference) | | NIST Chemistry Reference | 2-Aminobenzaldehyde(529-23-7) | | EPA Substance Registry System | Benzaldehyde, 2-amino- (529-23-7) |
| | 2-Aminobenzaldehyde Usage And Synthesis |
| Description | 2-Aminobenzaldehyde is one of the three isomers of aminobenzaldehyde. It is used as a versatile substrate for rhodium-catalyzed alkyne hydroacylation.1 It is used to prepare quinoline derivatives as antiviral agents, electroluminescent materials for OLEDs, and 2-tosylaminopheyl cyclopropylmethanols for gold-catalyzed cyclopropyl carbinol rearrangement. It is also used for the Friedländer-type synthesis, the benzyl C-H bond amination of acrymethylamines catalyzed by hydroxy-TEMPO, and for silver-catalyzed aniline mediated cascade hydroamination/cycloaddition reactions.
| | Physical properties | 2-Aminobenzaldehyde has recently been identifed as an important component in the fragrant scents of many flowers. These flowers include broom (Spartium junceum), false acacia (Robinia pseudoacacia), European bird cherry (Padus avium), ily (Lilium candidum), seringat (Philadelphus coronarius), Pittosporum tobira, Hypecoum imberbe, and H. fragrant. This chemical is also responsible for the“"sweet" or fragrant odor of the wild mushroom Hebeloma sacchariolens.
| | Uses | Reactant for:
Preparation of quinoline derivatives as antiviral agents
Preparation of electroluminescent materials for OLEDs
Friedlander-type synthesis
Preparation of 2-tosylaminophenyl cyclopropylmethanols for gold-catalyzed cyclopropyl carbinol rearrangement
Benzyl C-H bond amination of arylmethylamines catalyzed by hydroxy-TEMPO
Silver-catalyzed aniline mediated cascade hydroamination/cycloaddition reactions
| | Preparation | 2-Aminobenzaldehyde is prepared by reduction of 2- nitrobenzaldehyde with aqueous ferrous sulfate and ammonia. Since the product contains both aldehyde and amino groups it polymerizes easily. This means that it must be isolated rapidly after it forms, which is done by steam distillation of the product from the reaction mixture. | | Reference | M. Castaing, S. L. Wason, B. Estepa, J. F. Hooper, M. C. Willis, 2‐Aminobenzaldehydes as Versatile Substrates for Rhodium‐Catalyzed Alkyne Hydroacylation: Application to Dihydroquinolone Synthesis, Angewandte Chemie, 2013, vol. 52, pp. 13280-13283 | | Chemical Properties | light yellow crystalline powder | | Uses | Reactant for:
- Preparation of quinoline derivatives as antiviral agents
- Preparation of electroluminescent materials for OLEDs
- Friedlander-type synthesis
- Preparation of 2-tosylaminophenyl cyclopropylmethanols for gold-catalyzed cyclopropyl carbinol rearrangement
- Benzyl C-H bond amination of arylmethylamines catalyzed by hydroxy-TEMPO
- Silver-catalyzed aniline mediated cascade hydroamination/cycloaddition reactions
| | Purification Methods | Distil it in steam and recrystallise it from H2O or EtOH/ Et2O. The semicarbazone has m 247o. [Beilstein 14 H 21, 14 I 356, 14 II 14, 14 III 47, 14 IV 42.] |
| | 2-Aminobenzaldehyde Preparation Products And Raw materials |
|