|
|
| | 1,2,3,4-Tetrahydroquinoline Basic information |
| Product Name: | 1,2,3,4-Tetrahydroquinoline | | Synonyms: | 1,2,3,4-Tetrahydroquinoline, 95+%;1-AZATETRALIN;1,2,3,4-TETRAHYDROQUINOLINE;1,2,3,4-TETRAHYDRO-QUINOLINE HYDROCHLORIDE;AURORA KA-684;AKOS BBS-00003596;1 2 3 4-TETRAHYDROQUINOLINE 98+%;1,2,3,4-Tetrahydroquinoline99% | | CAS: | 635-46-1 | | MF: | C9H11N | | MW: | 133.19 | | EINECS: | 211-237-6 | | Product Categories: | Building Blocks;Chemical Synthesis;Heterocyclic Building Blocks;Alphabetical Listings;Flavors and Fragrances;Q-Z;Quinoline&Isoquinoline;Pyridines;Building Blocks;Heterocyclic Building Blocks;Isoquinolines;Quinolines;bc0001 | | Mol File: | 635-46-1.mol | 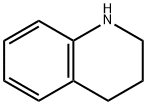 |
| | 1,2,3,4-Tetrahydroquinoline Chemical Properties |
| Melting point | 9-14 °C (lit.) | | Boiling point | 113-117 °C/10 mmHg (lit.)
249 °C (lit.) | | density | 1.061 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.593(lit.) | | Fp | 213 °F | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | form | Liquid | | pka | 5.09±0.20(Predicted) | | color | Clear pale yellow to yellow | | Odor | at 1.00 % in dipropylene glycol. honey civet animal phenolic | | PH | 10-11 (111g/l, H2O, 20℃)(as an emulsion) | | Odor Type | animal | | Water Solubility | <1 g/L (20 ºC) | | FreezingPoint | 11.0 to 20.0 ℃ | | BRN | 116149 | | LogP | 2.290 | | CAS DataBase Reference | 635-46-1(CAS DataBase Reference) | | NIST Chemistry Reference | Quinoline, 1,2,3,4-tetrahydro-(635-46-1) | | EPA Substance Registry System | 1,2,3,4-Tetrahydroquinoline (635-46-1) |
| | 1,2,3,4-Tetrahydroquinoline Usage And Synthesis |
| Chemical Properties | clear pale yellow to yellow liquid | | Uses | 1,2,3,4-Tetrahydroquinoline is a reagent used in the synthesis of N-substituted benzoyl-1,2,3,4-tetrahydroquinolyl-1-carboxamides displaying fungicidal activity. | | Definition | ChEBI: A member of the class of quinolines that is the 1,2,3,4-tetrahydro derivative of quinoline. | | Synthesis Reference(s) | Chemical and Pharmaceutical Bulletin, 34, p. 3905, 1986 DOI: 10.1248/cpb.34.3905
Tetrahedron, 52, p. 1631, 1996 DOI: 10.1016/0040-4020(95)00991-4 | | Synthesis | 1,2,3,4-Tetrahydroquinoline is synthesised using quinoline N-oxide as a raw material by chemical reaction. The specific synthesis steps are as follows:
General procedure: In a 1.5 mL reaction vial, B(C6F5)3 (0.025 mmol, 5.0 mol %) was dissolved in chloroform (0.60 mL), towhich diethylsilane (1.75 mmol, 3.5 equiv) was added. After shaking briefly, quinolines (1a-p, 0.50 mmol, 1.0equiv) was subsequently added to the above catalyst solution under argon atmosphere. The reaction mixturewas stirred at 25-65 oC for 6-24 h for the reaction of 1a-h, and at 25-100 oC for 2-24 h for the reaction of 1i-p,then allowed to cool down to room temperature and concentrated under reduced pressure to give the crudeproduct. This reaction mixture was then treated with 0.25 N HCl ethereal solution (7 mL) and stirred at roomtemperature for 1 h to give the solid residue, which was subsequently washed with ether. The solid residue wasthen dissolved or suspended in MeOH (1.0 mL) and neutralized with Na2CO3·H2O (0.5 g) at 0 oC. After stirringfor 2 h, MeOH was removed under reduced pressure, and the neutralized reaction residue was dissolved inCH2Cl2 and washed with brine (5 mL) and water (5 mL). The crude product was then obtained from the organicphase of CH2Cl2 solution and finally purified by column chromatography on silica gel to give 2a-h(EtOAc/Hexane = 1/9) and 2i-p (EtOAc/Hexane = 3/7).
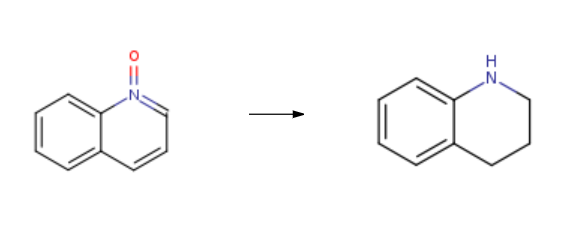
|
| | 1,2,3,4-Tetrahydroquinoline Preparation Products And Raw materials |
| Raw materials | Quinoline-->Ethylene glycol | | Preparation Products | 6-Bromoquinoline-->7-Amino-1-methyl-1,2,3,4-tetrahydroquinoline-->1,2,3,4-Tetrahydro-8-hydroxyquinoline-->Lilolidine-->7-NITRO-QUINOLINE-->4-Thiazolecarboxylic acid-->1-Piperidinecarboxylic acid, 4-(3,4-dihydro-1(2H)-quinolinyl)-, 1,1-dimethylethyl ester-->Quinoline, 8-ethyl-1,2,3,4-tetrahydro--->6-iodo-1,2,3,4-tetrahydroquinoline-->7-HYDROXY-5-OXO-N-PROPYL-2,3-DIHYDRO-1H,5H-PYRIDO[3,2,1-IJ]QUINOLINE-6-CARBOXAMIDE |
|