- Clotrimazole
-

- $20.00 / 1kg
-
2024-04-24
- CAS:23593-75-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons
- Clotrimazole
-

- $150.00 / 1Kg/Bag
-
2024-04-22
- CAS:23593-75-1
- Min. Order: 1Kg/Bag
- Purity: 0.99
- Supply Ability: 20 tons
- Clotrimazole
-

- $3.00 / 1kg
-
2024-04-12
- CAS:23593-75-1
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 10 tons
Related articles - What is clotrimazole used to treat?
- The imidazole antimycotic clotrimazole is used to treat mycoses of the skin induced or sustained by fungi such as dermatophyte....
- Mar 16,2024
|
| | Clotrimazole Chemical Properties |
| Melting point | 147-149°C | | Boiling point | 501.14°C (rough estimate) | | density | 1.1095 (rough estimate) | | refractive index | 1.5940 (estimate) | | storage temp. | 2-8°C | | solubility | Soluble in chloroform, DMSO, DMF and alcohols | | pka | pKa 4.7(EtOH 50%aq ) (Uncertain) | | color | White to Almost white | | Water Solubility | <10mg/L(25 ºC) | | λmax | 556nm(Phosphate buffer sol.)(lit.) | | Merck | 14,2417 | | InChI | InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H | | InChIKey | VNFPBHJOKIVQEB-UHFFFAOYSA-N | | SMILES | C1N(C(C2=CC=CC=C2Cl)(C2=CC=CC=C2)C2=CC=CC=C2)C=CN=1 | | NIST Chemistry Reference | Clotrimazole(23593-75-1) |
| | Clotrimazole Usage And Synthesis |
| Broad-spectrum antifungal drug | Clotrimazole is an artificially synthetic azole antifungal agent with inhibitory effect on a variety of pathogenic fungi. It is also able to directly kill the fungi at high concentration. Clotrimazole, through interfering with cytochrome P450 activity, inhibits the biosynthesis of the fungal ergosterol and other steroids; cause damage of the fungal cell membrane and change its permeability, causing the leakage of many kinds of important material in the cell; it can also inhibit the biosynthesis of the fungal triglyceride and phosphor-lipid; the product can still inhibit the activity of oxidase and peroxidase, causing the over-accumulation of peroxides in the cell, causing the degeneration and necrosis of the fungal subcellular structures. Clotrimazole has broad-spectrum antifungal activity with excellent anti-fungal activity against Epidermophyton, Trichophyton, aspergillosis, coloring fungi, Cryptococcus and Candida species. It also has certain antifungal effect against sporothrix schenckii,blastomycosis dermatitidis, Blastomyces coccidioides as well as Histoplasma.
It has good anti-fungal effect against Candida, Cryptococcus, Aspergillus, algae bacteria, dermatophytes, etc in both in vivo and in vitro. It efficacy in treating skin superficial mycoses is similar as griseofulvin while its efficacy in treating deep fungal disease is similar as amphotericin B. It has excellent efficacy in treating visceral pathogenic fungi such as Candida albicans, Cryptococcus neoformans, coccidioidomycosis and histoplasma. Its effect on the Candida albicans is stronger than nystatin. Fungi are not easy to evolve drug resistance to this kind of product. This product is easily absorbed orally and can be used for the treatment of systemic fungal infection such as aspergillosis fumigatus, candidiasis, cryptococcosis, coccidioidomycosis, histoplasmosis (dogs, cats) and fungal septicemia. For severe fungal infection, it is preferably to use it in combination with amphotericin B. Topically it can also be used for the treatment of superficial fungal infections such as ringworm and livestock comb body and hair tinea. However, it is invalid in treating tinea capitis. Clotrimazole also has certain efficacy in treating some kinds of Gram-positive bacteria and Trichomonas vaginalis. | | Pharmacokinetics | This product is rarely absorbed after oral administration. Adult, after oral administration of 3, has the peak of the plasma concentration being only 1.29 mg/L at 2 hour while 0.78 mg/L at 6 hours. Upon continuous administration, because of the induction effect of liver enzymes, the serum concentration can in turn decrease. The elimination half-life is 4.5 to 6 hours. Most of the products is metabolic inactivated in the liver and is further excreted by bile with only a small amount (less than 1% of dose) being subject to urine excretion in its prototype. The urine excrete were mostly inactive metabolites. This product is presented at high concentration in the feces including the non-absorption part of oral administration as well as the fraction via biliary excretion. Clotrimazole is widely distributed in the body with high concentration existing in the liver and adipose tissue. It can’t penetrate normal meninges and get into the cerebrospinal fluid. This product has a serum protein binding rate of 50%.
| | Clinical application | Clinical topical usage of 1% clotrimazole cream or solution for treating candidiasis ringworm, tinea versicolor, athlete's foot, nail ringworm and other skin infections. Clotrimazole vaginal tablets (100mg) administration can be used for treating candidiasis and trichomoniasis vaginal infections. Side effects include local irritation and burning sensations. Oral administration can be used for the treatment of deep fungal infection but with serious side effects such as gastrointestinal irritation, neutropenia, and liver function abnormalities.
1. Oral administration: (1) can be used for the treatment of oropharyngeal candidiasis. (2) it can also be used for chemotherapy and the prevention of the oropharynegeal candidiasis infection of immunocompromised or defect patients.
2. Topical: (1) skin candidiasis. (2) Tinea, jork itch and ringworm caused by Trichophyton, Epidermophyton and small spores; tinea versicolor caused by Pityrosporum.
3. Vaginal administration: (1) Vaginitis induced by Candida or other fungi. (2) vaginal infection caused by yeast. (3) Vagina super-infection caused by susceptible strains of the drug.
The above information is edited by the chemicalbook of Dai Xiongfeng. | | Mechanism | 1. Through reducing the activity of cytochrome P450, it inhibit the biosynthesis of ergosterol and other sterols contained in the fungal cell membrane, causing damage of the fungal cell membrane and change its permeability and causing important intracellular material leakage.
2. Clotrimazole can inhibit the biosynthesis of the fungal triglyceride and phospholipid.
3. It can still inhibit the activity of oxidase and peroxidase, leading to the over-accumulation of hydrogen peroxide inside the cell, causing fungal subcellular structures degeneration and necrosis.
4. For Candida albicans, clotrimazole can suppresses the process of its conversion from spores into invasive hyphae.
| | Drug Interactions | 1, the drug can inhibit the metabolism of drugs like sirolimus and dofetilide mediated by cytochrome P450 3A4 so that the plasma concentration of these drugs increases.
2, when being used in combination with tacrolimus and acamprosate, it can slow down the metabolism of these drugs and increasing its toxicity.
3, when clotrimazole is used in combination with betamethasone, it can make the skin be vulnerable to infection and increase the opportunities for microbial growth; the possible mechanism is through inhibiting the local inflammation.
4, when being used in combination with nystatin, amphotericin B and flucytosine, no synergistic antibacterial effect has been observed for inhibiting Candida albicans; moreover, it has antagonism effect in pharmacodynamic when being used in combination with amphotericin B.
| | Side effects | 1, Oral administration: (1) gastrointestinal reactions: including loss of appetite, nausea, vomiting, abdominal pain and diarrhea. (2) Liver toxicity: Because most of clotrimazole is subjecting to liver metabolism, it can lead to liver damage and causing increased level of serum bilirubin, alkaline phosphatase and aminotransferase. These symptoms can resume after drug withdrawal. (3) There are occasionally transient neuropsychiatric disorders, manifested as depression, hallucinations and disorientation and so on. Once such reactions occur, treatment must be immediately discontinued. (4) There may be occasionally leukopenia. (5) Some patients, after taking the drug, can get a burning sensation in the urethra. At this time, you should drink more water to ensure a high urine output. However, there is no need for stopping the drug.
2, Topical administration may occasionally cause local irritation, itching or burning sensation. Skin can get erythema, papules, vesicles, scaling and so on. There is also report about contact dermatitis.
3, a small amount of vaginal administrators may get vaginal burning sensation, abdominal cramping, and bloating, frequent urination and so on. There may also be varying degrees of allergic reactions such as skin redness and itching, shortness of breath, hypotension or transient feeling, nausea and diarrhea.
| | Administration instructions | 1, the drug can’t be used for systemic fungal infections. Owing to the poor oral absorption of the drug, adverse reactions are common while now we mostly apply topical or vaginal administration.
2, upon using this drug, you should avoid eye contact with the drug.
3, clotrimazole vaginal tablets should be prohibited through oral administration.
4, Administration of the tablets of this product once should overall take 15 to 30 minutes to allow the drug being slowly and completely dissolved. You should avoid chewing or swallowing whole tablet.
5, virginal treatment should be prohibited during menstruation.
6. if there are temporary neuropsychiatric disorder, severe gastrointestinal reactions and local skin allergic reaction happening, you should discontinue the drug immediately.
| | Chemical Properties | It is white powder or colorless crystalline powder. It has a melting point of 147-149 ℃. It is soluble in ethanol, acetone, and chloroform, but almost insoluble in water. It is odorless, tasteless and subject to rapid decomposition in an acid solution. Clotrimazole hydrochloride has a m.p. of 159 ℃.
| | Production method | It can be obtained from o-benzoic acid via esterification, addition, hydrolysis, chlorination, and condensation. It may also be obtained by chlorination of o-chlorotoluene into o-chloro benzotrichloride, which, in the presence of aluminum chloride, has condensation reaction with benzene to generate diphenyl-(2-chlorophenyl) methyl chloride, which finally has condensation reaction with imidazole to obtain clotrimazole.
| | Description | Clotrimazole is an imidazole antifungal that is active against a wide variety of fungal forms and is effective in many types of fungal infections. It tightly binds sterol 14-α demethylase isoform B from A. fumigatus (KD = 103 nM) and, like other imidazoles, disturbs the fungal cell membrane. In mammalian cells, clotrimazole potently inhibits the calcium-dependent potassium channels Kv1.3 and IK-1 (IC50 = 6.0 and 0.07 μM, respectively). | | Chemical Properties | White or pale yellow, crystalline powder. | | Originator | Canesten,Bayer,UK,1973 | | Uses | Clotrimazole formally also is an imidazole derivative because of the presence of an imi�dazole ring in its structure. It is believed that, like miconazole, econazole, and other “pure”
representatives of the imidazole class, it also inhibits the biosynthesis of ergosterin in the
cytoplasmatic membrane of fungi.
In terms of pharmacological action, clotrimazole is very similar to miconazole. It has a
broad spectrum of antifungal activity. It is effective with respect to dermatophytes, and it
also has an antimicrobial effect against streptococci and staphylococci. It is also effective
with respect to trichomonases. It is very widely used, both externally and vaginally for
treating superficial infections. Synonyms of this drug are canesten, empecid, lotrimin,
micosporin, and others. | | Uses | An antifungal compound and CYP inhibitor.
Clotrimazole (Lotrimin, Mycelex, etc.) is a synthetic imidazole agent. It acts by altering cell membrane permeability. It inhibits the growth of most dermatophyte species, yeast and Malassezia furfur. | | Uses | Clotrimazole is an antifungal agent. | | Definition | ChEBI: A member of the class of imidazoles that is 1H-imidazole in which the hydrogen attached to a nitrogen is replaced by a monochlorotrityl group. | | Indications | Clotrimazole (Lotrimin, Gyne-Lotrimin, Mycelex) is a
broad-spectrum fungistatic imidazole drug used in the
topical treatment of oral, skin, and vaginal infections
with C. albicans. It is also employed in the treatment of
infections with cutaneous dermatophytes.
Topical use results in therapeutic drug concentrations
in the epidermis and mucous membranes; less
than 10% of the drug is systemically absorbed. Although
clotrimazole is generally well tolerated, local abdominal
cramping, increased urination, and transient
liver enzyme elevations have been reported. | | Manufacturing Process | 156.5 g (0.5 mol) o-chlorophenyldiphenylmethyl chloride and 34 g (0.5 mol)
imidazole are dissolved in 500 ml acetonitrile, with stirring, and 51 g (0.5
mol) triethylamine are added, whereupon separation of triethylamine
hydrochloride occurs even at room temperature. In order to complete the
reaction, heating at 50°C is carried out for 3 hours. After cooling, one liter of
benzene is added and the reaction mixture is stirred, then washed salt-free
with water. The benzene solution is dried over anhydrous sodium sulfate,
filtered and concentrated by evaporation; giving 167 g crude 1-(o�chlorophenylbisphenylmethyl)-imidazole. By recrystallization from acetone,
115 g (= 71% of the theory) of pure 1-(o-chlorophenylbisphenylmethyl)-
imidazole of MP 154° to 156°C are obtained. | | Brand name | Gyne-
Lotrimin (Schering-Plough); Gynix (Teva); Lotrimin
(Schering-Plough); Mycelex (Bayer). | | Therapeutic Function | Antifungal | | General Description | Chemical structure: imidazole | | Biochem/physiol Actions | Clotrimazole is a specific inhibitor of Ca2+-activated K+ channels. It is an antifungal azole. Clotrimazole is a derivative of imidazole and has similar antimicrobial action and activity to ketoconazole. It inhibits cytochrome P450-dependent 14α-demethylase, which is critical to ergosterol biosynthesis. The accumulated 14α-methylated sterols change the membrane structure of sensitive fungi, altering cell membrane permeability. | | Clinical Use | 1-(o-Chloro-α,α-diphenylbenzyl)imidazole (Lotrimin, Gyne-Lotrimin, Mycelex) is a broad-spectrum antifungal drug thatis used topically for the treatment of tinea infections and candidiasis.It occurs as a white crystalline solid that is sparinglysoluble in water but soluble in alcohol and most organic solvents.It is a weak base that can be solubilized by dilute mineralacids.
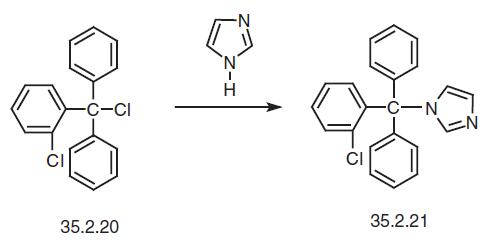
Clotrimazole is available as a solution in polyethyleneglycol 400, a lotion, and a cream in a concentration of 1%.These are all indicated for the treatment of tinea pedis, tineacruris, tinea capitis, tinea versicolor, or cutaneous candidiasis.A 1% vaginal cream and tablets of 100 mg and 500 mgare available for vulvovaginal candidiasis. Clotrimazole isextremely stable, with a shelf life of more than 5 years. | | Synthesis | Clotrimazole, 1-(o-chloro-α,α-diphenylbenzyl)imidazole (35.2.21), is syn�thesized by reacting 2-chlorotriphenylmethylchloride (35.2.20) with imidazole in the pres�ence of triethylamine.
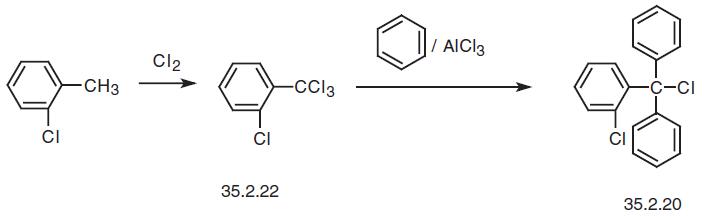
The starting substance 2-chlorotriphenylmethylchloride is made in various ways. In par�ticular, chlorinating 2-chlorotoluene under light makes 2-chlorotrichloromethylbenzene (35.2.22), which is reacted with benzene in the presence of aluminum chloride to give 2-chlorotriphenylmethylchloride (35.2.20).
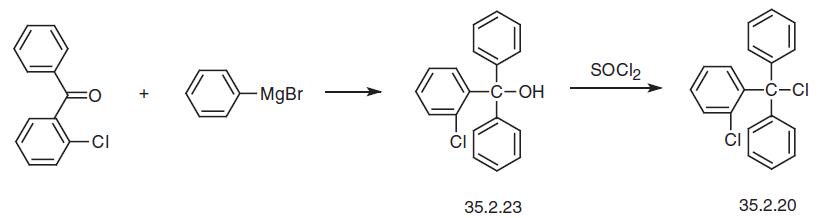
An alternative way of making 2-chlorotriphenylmethylchloride is a Grignard reaction between 2-chlorobenzolphenone and phenylmagnesium bromide, followed by substitution of the hydroxyl group in the resulting 2-chlorotriphenylmethylcarbinol (35.2.23) with a chlorine using thionyl chloride.
And finally, reacting phosphorous pentachloride with 2-chlorobenzophenone gives 2�- chloro-1,1-dichlorodiphenylmethane (35.2.24), which is used for the alkylation of benzene in the presence of aluminum chloride and gives 2-chlorotriphenylmethylchloride (35.2.20).
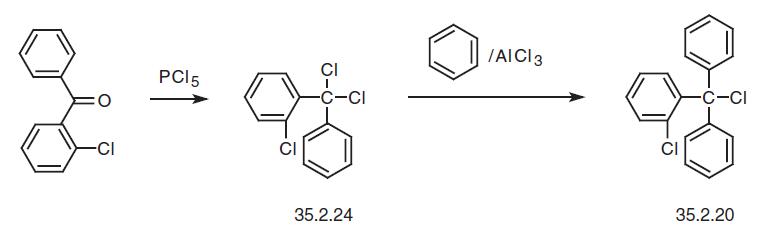 | | Veterinary Drugs and Treatments | Topical clotrimazole has activity against dermatophytes and yeasts; it may be useful for localized lesions associated with Malassezia. It is
not very effective in treating dermatophytosis in cats.
Clotrimazole inhibits the biosynthesis of ergosterol, a component of fungal cell membranes leading to increased membrane permeability
and probable disruption of membrane enzyme systems. | | storage | Store at RT |
| | Clotrimazole Preparation Products And Raw materials |
|