- Labetalol
-

- $15.00 / 1KG
-
2021-07-13
- CAS:36894-69-6
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Labetalol
-

- $15.00 / 1KG
-
2021-07-10
- CAS:36894-69-6
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| Product Name: | Labetalol | | Synonyms: | 5-[1-HYDROXY-2-[(1-METHYL-3-PHENYLPROPYLAMINO)ETHYL]]SALICYLAMIDE;LABETALOL;LABETOLOL;5-[1-hydroxy-2-[(1-methyl-3-phenylpropylamino)ethyl]saicylamide;Labetalol (base and/or unspecified salts);SeH-15719W;2-hydroxy-5-[(1S)-1-hydroxy-2-{[(2R)-4-phenylbutan-2-yl]aMino}ethyl]benzaMide;Laβlol | | CAS: | 36894-69-6 | | MF: | C19H24N2O3 | | MW: | 328.41 | | EINECS: | 253-258-3 | | Product Categories: | API's | | Mol File: | 36894-69-6.mol | 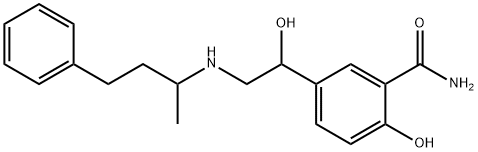 |
| | Labetalol Chemical Properties |
| | Labetalol Usage And Synthesis |
| Use advice | Labetalol is a competitive α1- and β-antagonist which is more active at β-
than at α-receptors (1 : 3–1 : 7, depending on route). It may be administered
orally or i.v. Intravenous bolus doses range from 50–200mg, with infusion
rates between 5–150mgh –1, titrated to effect. | | Description | Labetalol is an α-adrenergic and α-1 blocking agent
which caused contact dermatitis and a contact anaphylactoid
reaction during patch testing in a nurse. | | Originator | Trandate,Allen and Hanburys,UK,1977 | | Uses | Labetalol is used to treat essential hypertension. | | Uses | Anti-adrenergic (α-receptor); anti-adrenergic (β-receptor). | | Definition | ChEBI: A secondary amino compound formally derived from ammonia by replacing two of the hydrogens by 2-(3-carbamoyl-4-hydroxyphenyl)-2-hydroxyethyl and 4-phenylbutan-2-yl groups. It is an adrenergic antagonist used to treat high blood pressure. | | Manufacturing Process | (a) 5-Bromoacetylsalicylamide (2.6 g), N-benzyl-N-(1-methyl-3-phenylpropyl)
amine (4.8 g) and methyl ethyl ketone (50 ml) were heated at reflux for 40
minutes. The solvent was removed and the residue was treated with benzene.
The secondary amine hydrobromide was filtered off and discarded, and the
filtrate was evaporated to dryness. The residue was treated with an excess of
ethanolic hydrogen chloride when 5-[N-benzyl-N-(1-methyl-3-phenylpropyl)-
glycyl]-salicylamide hydrochloride (1.15 g) crystallized out, MP 139°C to
141°C.
(b) 5-[N-benzyl-N-(1-methyl-3-phenylpropyl)glycyl]-salicylamide
hydrochloride (0.75 g), 10% mixture of PdO and PtO on carbon catalyst (0.1
g) and ethanol (20 ml) were shaken at room temperature and pressure with
hydrogen until uptake ceased. The catalyst was filtered off and the filtrate
evaporated to dryness. The residue was crystallized from ethanol to give 5-[1-
hydroxy-2-(1-methyl-3-phenylpropyl)aminoethyl]salicylamide hydrochloride as
a white solid (0.40 g), MP 188°C. | | Brand name | Normodyne (Schering); Trandate (Promethus). | | Therapeutic Function | Alpha-adrenergic blocker, Beta-adrenergic blocker | | Biological Functions | Labetalol (Normodyne, Trandate) possesses both -
blocking and β-blocking activity and is approximately
one-third as potent as propranolol as a -blocker and
one-tenth as potent as phentolamine as an -blocker.
The ratio of β- to α-activity is about 3:1 when labetalol
is administered orally and about 7: 1 when it is administered
intravenously. Thus the drug can be most conveniently
thought of as a β -blocker with some -blocking
properties. | | General Description | Labetalol is a phenylethanolamine derivative, is representative of a classof drugs that act as competitive blockers at α1-, β1-, andβ2-receptors. It is a more potent β-blocker than α-blocker.Because it has two asymmetric carbon atoms (1 and 1' ), it existsas a mixture of four isomers. It is this mixture that is usedclinically in treating hypertension. The different isomers,however, possess different α- and β-blocking activities. The -blocking activity resides solely in the (1R,1 'R) isomer,whereas the 1-blocking activity is seen in the (1S,1 R) and(1S,1'S) isomers, with the (1S,1'R) isomer possessing thegreater therapeutic activity. | | Contact allergens | This beta-adrenergic and alpha-1 blocking agent
caused contact dermatitis and a contact anaphylactoid
reaction during patch testing in a nurse. | | Mechanism of action | Labetalol produces equilibrium-competitive antagonism
at β-receptors but does not exhibit selectivity for β1- or β2-receptors. Like certain other β-blockers (e.g., pindolol
and timolol), labetalol possesses some degree of
intrinsic activity. This intrinsic activity, or partial agonism,
especially at β2-receptors in the vasculature, has
been suggested to contribute to the vasodilator effect
of the drug. The membrane-stabilizing effect, or local
anesthetic action, of propranolol and several other β-blockers, is also possessed by labetalol, and in fact the
drug is a reasonably potent local anesthetic.
Labetalol appears to produce relaxation of vascular
smooth muscle not only by α-blockade but also by a
partial agonist effect at β2-receptors. In addition, labetalol
may produce vascular relaxation by a direct
non–receptor-mediated effect.
Labetalol can block the neuronal uptake of norepinephrine
and other catecholamines. This action, plus its
slight intrinsic activity at α-receptors, may account for
the seemingly paradoxical, although infrequent, increase
in blood pressure seen on its initial administration. | | Pharmacokinetics | Labetalol is almost completely absorbed from the gastrointestinal
tract. However, it is subject to considerable
first-pass metabolism, which occurs in both the gastrointestinal
tract and the liver, so that only about 25%
of an administered dose reaches the systemic circulation.
While traces of unchanged labetalol are recovered
in the urine, most of the drug is metabolized to inactive
glucuronide conjugates.The plasma half-life of labetalol
is 6 to 8 hours, and the elimination kinetics are essentially
unchanged in patients with impaired renal failure. | | Clinical Use | Labetalol is a clinically usefulantihypertensive agent. The rationale for its use in themanagement of hypertension is that its α-receptor–blockingeffects produce vasodilation and its β-receptor–blockingeffects prevent the reflex tachycardia usually associated withvasodilation. Although labetalol is very well absorbed, it undergoesextensive first-pass metabolism. | | Side effects | There have been reports of excessive hypotension and
paradoxical pressor effects following intravenous administration
of labetalol. These latter effects may be
due to a labetalol-induced blockade of neuronal amine
uptake, which increases the concentrations of norepinephrine
in the vicinity of its receptors.
Approximately 5% of the patients who receive labetalol
complain of side effects typical of noradrenergic
nervous system suppression. These include postural hypotension,
gastrointestinal distress, tiredness, sexual
dysfunction, and tingling of the scalp. Most of these effects
are related to α-blockade, although the tingling of
the scalp may be due to the drug’s intrinsic activity at α-receptors. Side effects associated with β-blockade, such
as induction of bronchospasm and congestive heart failure,
may also occur, but generally at a lower frequency
than -receptor–associated effects.
Skin rashes have been reported, as has an increase
in the titer of antinuclear antibodies. Despite the latter
observation, the appearance of a systemic lupus syndrome
is rare. Labetalol also has been reported to interfere
with chemical measurements of catecholamines
and metabolites. | | Synthesis | Labetalol, 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropanol)amino)]
ethyl] benzamide (12.1.12) is synthesized by the N-alkylation of N-benzyl-N(4-phenyl-2-
butyl)amine 5-bromacetylsalicylamide and forming aminoketone (12.1.11), which is fur�ther debenzylated by hydrogen using a palladium¨Cplatinum on carbon catalyst into
labetalol (12.1.12) [28¨C30]. 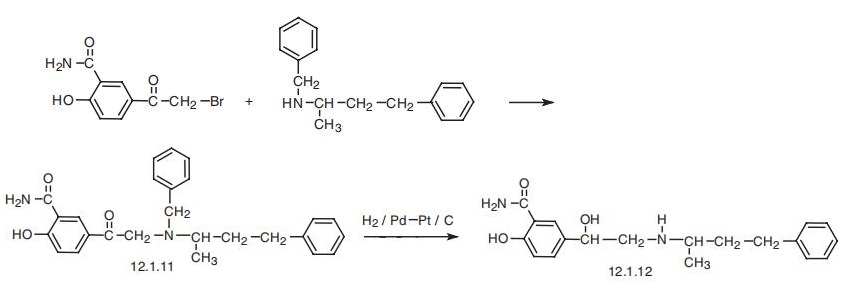
|
| | Labetalol Preparation Products And Raw materials |
|