- 2,3-Dihydroxynaphthalene
-
- $100.00 / 1KG
-
2025-09-25
- CAS:92-44-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
|
| | 2,3-Dihydroxynaphthalene Basic information |
| Product Name: | 2,3-Dihydroxynaphthalene | | Synonyms: | 2,3-Dihydroxynapthalene;Naphthalenediol-(2,3);2,3-DIHYDROXYNAPHTHALENE;2,3-NAPHTHALENEDIOL;2,3-Dihydroxynaphthylene;2,3-dihydrroxynaphthalene;2,3-Dihydroxynaphthalene 1g [92-44-4];2,3-Dihydroxynaphthalene, 98% 50GR | | CAS: | 92-44-4 | | MF: | C10H8O2 | | MW: | 160.17 | | EINECS: | 202-156-7 | | Product Categories: | Cell Biology;Chemical Synthesis;DIG-DY;Organic Building Blocks;Oxygen Compounds;Polyols;Aromatics;Naphthalene derivatives;Bioactive Small Molecules;Building Blocks | | Mol File: | 92-44-4.mol | 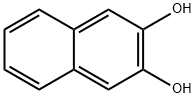 |
| | 2,3-Dihydroxynaphthalene Chemical Properties |
| Melting point | 161-165 °C (lit.)
162-164 °C | | Boiling point | 246.06°C (rough estimate) | | density | 1.0924 (rough estimate) | | bulk density | 500kg/m3 | | vapor pressure | 0.8 hPa (149 °C) | | refractive index | 1.5178 (estimate) | | Fp | 175 °C | | storage temp. | Store below +30°C. | | solubility | 4g/l | | pka | 9.10±0.40(Predicted) | | form | Powder | | color | White to pinkish to beige-grayish | | PH | 6 (1g/l, H2O, 20℃) | | Water Solubility | slightly soluble | | BRN | 742375 | | Cosmetics Ingredients Functions | HAIR DYEING | | Cosmetic Ingredient Review (CIR) | 2,3-Dihydroxynaphthalene (92-44-4) | | InChI | 1S/C10H8O2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6,11-12H | | InChIKey | JRNGUTKWMSBIBF-UHFFFAOYSA-N | | SMILES | Oc1cc2ccccc2cc1O | | CAS DataBase Reference | 92-44-4(CAS DataBase Reference) | | NIST Chemistry Reference | 2,3-Naphthalenediol(92-44-4) | | EPA Substance Registry System | 2,3-Naphthalenediol (92-44-4) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-37/39 | | WGK Germany | 3 | | RTECS | QJ4750000 | | F | 10-23 | | Autoignition Temperature | >330 °C | | TSCA | TSCA listed | | HS Code | 29072900 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Acute Tox. 4 Oral
Eye Dam. 1 | | Toxicity | LD50 orally in Rabbit: 970 mg/kg |
| | 2,3-Dihydroxynaphthalene Usage And Synthesis |
| Chemical Properties | Off white to redish white powder. 2,3-Naphthalenediol [92-44-4], mp 161℃, gives a dark blue
color with iron(III) chloride; is aminated to 2-
amino-3-naphthol at 140℃ and 2,3-naphthalenediamine at 240℃; and couples with diazotized anilines in the 1- or 1,4-positions. | | Uses | 2,3-Dihydroxynaphthalene is used in making dyes, pigments, fluorescent whiteners, tanning agents, antioxidants, and antiseptics. | | Uses | 2,3-Naphthalenediol is used in cosmetics asa component of oxidative hair dye. It is usedat a concentration of <0.1%. | | Uses | 2,3-Dihydroxynaphthalene may be used in the following studies:
- Construction of dinaphtho[2,1-b;2′,3′-d]furan-6-ol, via dehydration reaction in the presence of strong acid.
- As fused ring catecholate type ligand for the surface modification of nanocrystalline TiO2 particles.
- As adsorptive and competing ligand during the chemical speciation of iron in seawater by cathodic stripping voltammetry.
- Synthesis of cyclotriphosphazene derivatives, used as non-halogen flame retardants
| | Definition | ChEBI: Naphthalene-2,3-diol is a naphthalenediol. | | General Description | 2,3-Dihydroxynaphthalene is a polyhydroxy phenol. It is an aromatic dihydroxy compound having hydroxyl groups at ortho positions. Its reaction with molybdenum(VI) complexes has been reported. The asymmetric oxidative coupling polymerization of 2,3-dihydroxynaphthalene using the Cu(I)-bisoxazoline complex as catalyst has been reported to afford poly(2,3-dihydroxy-1,4-naphthylene), having a continuous 1,1′-bi-2-naphthol main chain structure. The nitrodisplacement reaction between nitrophthalodinitriles and 2,3-dihydroxynaphthalene has been investigated. | | Health Hazard | 2,3-Naphthalenediol shows low toxicity andmild irritant actions on the skin and eyes. Theoral LD50 value for rats of a 5% solutionin propylene glycol may be on the orderof 675 mg/kg (calculated by the method ofWeil). The intravenous LD50 value for miceis 56 mg/kg. At 1% concentration, it causedslight eye irritation in female albino rabbitsand exhibited erythemal response in guineapigs. At the 0.1% level (concentration in hairdye), it had no reaction on human skin. Thiscompound was nonmutagenic in Salmonellatyphimurium strain tests.
There is very little information in the literatureon the toxicity of 2,3-naphthalenediol.Assessment by the CIR expert panel on thesafety of this compound as used in cosmeticshas been inconclusive (Cosmetic, Toiletryand Fragrance Association 1987b). | | Synthesis | 2,3-Dihydroxynaphthalene is prepared by caustic fusion of 3-hydroxynaphthalene-2,6-disulfonic acid followed by treatment with dilute sulfuric acid under pressure, or by acid desulfonation of 2,3-dihydroxynaphthalene-6-sulfonic acid. |
| | 2,3-Dihydroxynaphthalene Preparation Products And Raw materials |
| Raw materials | 2H-naphtho[2,3-d][1,3]dioxole-->(1aS,1bR,2aS,6bR)-1a,1b,2a,6b-Tetrahydronaphtho[1,2-b:3,4-b']bisoxirene-->Naphthalene-2,3-diol diacetate | | Preparation Products | 2,3-diaminonaphthalene-->4 5-DICHLOROCATECHOL 97-->3-METHOXY-2-NAPHTHOL 97-->p-Toluic acid-->2,3-DICHLORONAPHTHALENE-->3-AMINO-2-NAPHTHOL-->6,13-dihydrodibenzo[b,i]phenazine |
|