| Company Name: |
Zhejiang Huida Biotech Co., LTD
|
| Tel: |
0571-89903882 13626641628 |
| Email: |
jiangnan@huidabiotech.com |
| Products Intro: |
Product Name:Flavomycoin
CAS:77814-07-4
Purity:99% HPLC Package:10mg;100mg;1g;10g;100g
|
| Company Name: |
Zhejiang Huida Biotech Co., LTD
|
| Tel: |
0571-0571-89903882 15990081639 |
| Email: |
sunshixuan@huidabiotech.com |
| Products Intro: |
Product Name:Flavomycoin
CAS:77814-07-4
Purity:99% HPLC Package:10mg;100mg;1g;10g;100g
|
|
| | roflamycoin Basic information |
| Product Name: | roflamycoin | | Synonyms: | 14,37-Dioxabicyclo[31.3.1]heptatriaconta-3,5,7,9,11-pentaen-13-one, 19,21,23,25,27,29,31,33,35-nonahydroxy-12,16-dimethyl-15-(1-methylethyl)-, (1R,3E,5E,7E,9E,11E,15S,16S,19S,21S,23S,25R,27R,29S,31R,33S,35S)- (9CI);Roflamycoin | | CAS: | 77814-07-4 | | MF: | C40H66O12 | | MW: | 738.94 | | EINECS: | | | Product Categories: | | | Mol File: | 77814-07-4.mol | 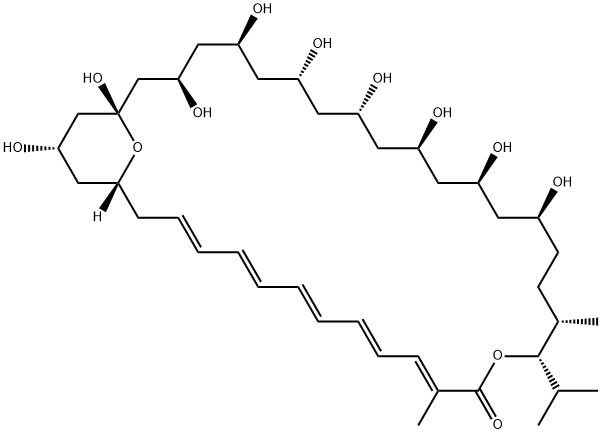 |
| | roflamycoin Chemical Properties |
| Boiling point | 972.8±65.0 °C(Predicted) | | density | 1.154±0.06 g/cm3(Predicted) | | pka | 12.67±0.70(Predicted) |
| | roflamycoin Usage And Synthesis |
| Synthesis | For Rychnovsky and co-worker’s synthesis of roflamycoin (45), the
hemiketal-pyran linkage required differential substitution (Scheme 9) and
demonstrated the power of the sequential double addition strategy.14
Treatment of bis-epoxide S,S-4 with a stoichiometric amount of
(benzyloxy)methyllithium 38 and BF3?OEt2 in THF at –78 °C provided monoaddition adduct 39, which was then treated with the organolithium formed
from transmetallation of 2,2-bis-(tributyltin)dithiane 40. This single-pot
procedure afforded dithiane diol 41 in 56% overall yield. Subsequent
acetonide formation and transmetallation provided a competent nucleophile
for direct displacement of dibromide 42, which itself was derived from
dichlorodiol R,R-3. The resulting bis-acetonide (43) was obtained in 60%
overall yield and provided a rapid access to tris-acetonide 44, a key
intermediate for the synthesis of the hemiketal-pyran moiety of 45. Similar
sequential addition strategies have proven to be useful as well.
 |
| | roflamycoin Preparation Products And Raw materials |
|