| | apomorphine Basic information |
| Product Name: | apomorphine | | Synonyms: | apomorphine;(6AR)-6-METHYL-5,6,6A,7-TETRAHYDRO-4H-DIBENZO[DE,G]QUINOLINE-10,11-DIOL OR APOMORPHINE;(6aS)-5,6,6aα,7-Tetrahydro-6-methyl-4H-dibenzo[de,g]quinoline-10,11-diol;(6aR)-5,6,6a,7-Tetrahydro-6-methyl-4H-dibenzo[de,g]quinoline-10,11-diol;6aβ-Aporphine-10,11-diol;Apomorphin;ApoMorphine-d3;4H-Dibenzo[de,g]quinoline-10,11-diol,5,6,6a,7-tetrahydro-6-Methyl-, (6aR)- | | CAS: | 58-00-4 | | MF: | C17H17NO2 | | MW: | 267.32 | | EINECS: | 200-360-0 | | Product Categories: | | | Mol File: | 58-00-4.mol | 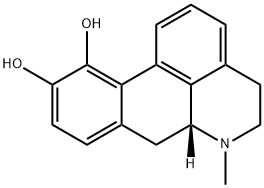 |
| | apomorphine Chemical Properties |
| Melting point | 195°C (rough estimate) | | Boiling point | 410.51°C (rough estimate) | | density | 1.0690 (rough estimate) | | refractive index | 1.5400 (estimate) | | solubility | Aqueous Base (Slightly), DMSO (Slightly) | | pka | 7.0, 8.92(at 25℃) | | form | Solid | | color | White, crystalline alkaloid | | Water Solubility | 20g/L(25 ºC) | | Stability: | Hygroscopic |
| | apomorphine Usage And Synthesis |
| Chemical Properties | Alkaloid; white crystalline mass; turns
green on exposure; weakly soluble in water. | | Originator | Apomorphine
hydrochloride,Nastech
Pharmaceuticals
Company, Inc. | | Uses | To treat acute poisoning; in the diagnosis and
treatment of parkinsonism; a weak sensitizer and a
powerful emetic. | | Definition | A derivative of mor-
phine that is a dopamine D2 agonist. | | Indications | Apomorphine (Uprima) is a short-acting central and peripheral
dopamine receptor agonist that can elicit male
sexual responses. Dopamine appears to have an important
role in normal erectile function. Apomorphine is a
D1-like,D2-like dopamine receptor agonist.Apomorphine
is not a new drug, and it has been used with limited success
in ameliorating the symptoms of Parkinson’s disease
and to induce emesis. It is not orally active except for a
special buccal formulation, but it can be given parenterally,
usually subcutaneously. Apomorphine is rapidly
cleared from the kidney because of its high lipid solubility,
its large volume of distribution, and its rapid metabolism. | | Manufacturing Process | 2 Methods of producing of apomorphine
1. The apomorphine was obtained by dehydratation of morphine at heating to
120°C in the presence phosphoric acid and rendering of HCl gas over reaction
mixture.
2. The morphine was converted to β-chloromorphine and then to
dichlorodihydrodesoxymorphine at heating to 140°-150°C in the presence
hydrochloric acid. Then apomorphine is obtained by dehydratation of
dichlorodihydrodesoxymorphine. | | Brand name | Apokyn
(Vernalis). | | Therapeutic Function | Emetic, Expectorant, Hypnotic, Antiparkinsonian,
Dopamine agonist | | Synthesis Reference(s) | Journal of the American Chemical Society, 72, p. 494, 1950 DOI: 10.1021/ja01157a129 | | Hazard | Poison; central nervous system effects. | | Clinical Use | Aside from sildenafil, apomorphine is one of the few
orally active (buccal route) pharmacological agents
used in the treatment of ED. Apomorphine stimulates
penile erection in both normal men and in men who are
impotent. Apomorphine can be the drug of choice in
patients with coexisting benign prostatic hyperplasia
(BPH), coronary artery disease, and hypertension. | | Side effects | When formulated into a controlled release sublingual
capsule, apomorphine becomes a very effective
orally active drug representative of a new class of centrally
acting drugs useful in the treatment of ED. It has
a narrow range (2 to 6 mg) of effective doses for its
erectogenic actions, with the higher doses being more
effective in inducing erections.Apomorphine can cause
nausea, emesis, drowsiness, and dizziness. | | Safety Profile | Poison by ingestion,subcutaneous, intravenous, and intraperitoneal routes.Experimental reproductive effects. Central nervous systemeffects. A powerful emetic. A weak sensitizer and maycause contact dermatitis. When heated to decomposition itemits hig | | Purification Methods | Crystallise R-apomorphine from CHCl3 and a little pet ether, also from Et2O with 1 mol of Et2O which it loses at 100o. It sublimes in a high vacuum. It is white but turns green in moist air or in alkaline solution. UV: max 336, 399 (98% EtOH). The di-O-methylether is an oil b 175o/high vacuum, whose picrate crystallises from MeOH and has m 140o (dec). The di-O-acetate crystallises from EtOAc/pet ether with m 127-128o, [] D -88o (c 1, 0.1 N HCl). The di-O-benzoyl derivative has m 156-158o (from EtOH) and []D +43.44o (c 3.3, CHCl3). [Pachorr et al. Chem Ber 35 4377 1902, Beilstein 21 H 246.] NARCOTIC. |
| | apomorphine Preparation Products And Raw materials |
|