| Company Name: |
Merck KGaA
|
| Tel: |
21-20338288 |
| Email: |
ordercn@merckgroup.com |
| Products Intro: |
Product Name:2-[(TRIMETHYLSILYL)METHYL]-2-PROPEN-1-OL
CAS:81302-80-9
|
|
| | 2-(trimethylsilylmethyl)allyl alcohol Basic information |
| | 2-(trimethylsilylmethyl)allyl alcohol Chemical Properties |
| solubility | Insol water; sol all organic liquids. |
| | 2-(trimethylsilylmethyl)allyl alcohol Usage And Synthesis |
| Physical properties | bp 54–56 °C/2 mmHg; nD
20 1.454; d 0.861 g
cm?3; fp 71?C. | | Uses | 2-Trimethylsilylmethyl-2-propen-1-ol reagent has been employed
as a conjunctive reagent which is considered to be a synthetic
equivalent of a zwitterionic, bifunctional compound possessing
a nucleophilic allylic anion synthon and an electrophilic cation
synthon in the same molecule. Derivatives function as trimethylenemethane (TMM) precursors
and undergo cyclopentannulation reactions; methylenecyclopentanes;
2-acetoxymethyl-3-trimethylsilylpropene; [3+2]
annulation; Methylenecyclopentane Annulation,Cyclocontraction-Spiroannulation, three-carbon condensative expansion;
cyclocontraction–spiroannulation. | | Preparation | 2-Trimethylsilylmethyl-2-propen-1-ol is prepared in four steps from methallyl
alcohol. The synthesis begins with the metalation with nbutyllithium
(2 equiv) producing the dianion which is bissilylated
by trapping with chlorotrimethylsilane to generate the
allylsilane. The TMS ether is subsequently removed by hydrolysis
(eq 1). The primary allylic alcohol serves as a precursor to
more functionalized reagents. 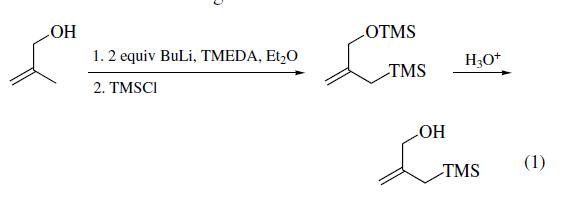
Useful derivatives of the alcohol are the primary allylic chloride,
mesylate, and acetate. These are easily prepared in a short number
of steps as shown (eq 2) or by functionalization of the primary
allylic alcohol. | | storage | flammable liquid; irritant; store at 0-4 °C. |
| | 2-(trimethylsilylmethyl)allyl alcohol Preparation Products And Raw materials |
|