|
|
| | (3β,7R,16E)-16,17-Didehydro-9-hydroxy-17-methoxy-2-oxocorynoxan-16-carboxylic acid methyl ester Basic information |
| Product Name: | (3β,7R,16E)-16,17-Didehydro-9-hydroxy-17-methoxy-2-oxocorynoxan-16-carboxylic acid methyl ester | | Synonyms: | (3β,7R,16E)-16,17-Didehydro-9-hydroxy-17-methoxy-2-oxocorynoxan-16-carboxylic acid methyl ester;Speciofoline;Spiro[3H-indole-3,1'(5'H)-indolizine]-7'-acetic acid, 6'-ethyl-1,2,2',3',6',7',8',8'a-octahydro-4-hydroxy-α-(methoxymethylene)-2-oxo-, methyl ester, (αE,1'R,6'S,7'S,8'aR)- | | CAS: | 5171-42-6 | | MF: | C22H28N2O5 | | MW: | 400.47 | | EINECS: | | | Product Categories: | | | Mol File: | 5171-42-6.mol | 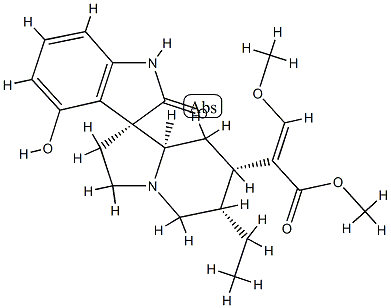 |
| | (3β,7R,16E)-16,17-Didehydro-9-hydroxy-17-methoxy-2-oxocorynoxan-16-carboxylic acid methyl ester Chemical Properties |
| | (3β,7R,16E)-16,17-Didehydro-9-hydroxy-17-methoxy-2-oxocorynoxan-16-carboxylic acid methyl ester Usage And Synthesis |
| Description | The leaves of Mitragyna speciosa Korth contain this alkaloid which crystallizes
from EtOH in colourless needles. It is laevorotatory with [α]22D - 103° (c 2.0,
CHCI3). The ultraviolet spectrum has absorption maxima at 223, 242 and
290 mJ.1. The alkaloid has been shown to be a stereoisomer of rotundifoline (q.v.). | | References | Beckett, Lee, Tackie., Tetrahedron Lett., 1709 (1963) |
| | (3β,7R,16E)-16,17-Didehydro-9-hydroxy-17-methoxy-2-oxocorynoxan-16-carboxylic acid methyl ester Preparation Products And Raw materials |
|