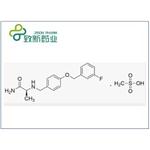
- Safinamide Mesylate
-
- $0.00 / 25kg
-
2023-12-01
- CAS:202825-46-5
- Min. Order: 1kg
- Purity: >99% by HPLC
- Supply Ability: 100kg/month
- Safinamide mesylate
-

- $10.00 / 1Kg/Drum
-
2023-03-06
- CAS:202825-46-5
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 10 ton
|
| | Safinamide mesylate Basic information |
| | Safinamide mesylate Chemical Properties |
| Melting point | 210° (dec) | | alpha | D25 +12.9° (c = 1.1% in 98% acetic acid) | | storage temp. | 2-8°C | | solubility | H2O: ≥15mg/mL | | form | powder | | color | white to tan | | optical activity | [α]/D +9.5 to +14°, c = 1 (95% acetic acid) |
| RIDADR | UN 2811 6.1 / PGIII | | WGK Germany | 3 |
| | Safinamide mesylate Usage And Synthesis |
| Description | Safinamide
methanesulfonate was approved in February 2015 by the
EMA for the treatment of mid- to late-stage fluctuating
Parkinson’s disease. This approval included use of the drug as
an add-on therapy for use with levodopa, either alone or in
combination with other existing therapies for Parkinson’s
disease.51 Safinamide methanesulfonate, an oral α-aminoamide
originally discovered by Farmitalia Carlo Erba and later
developed by Newron/Zambon, functions as a highly selective
and reversible inhibitor of MAO-B, leading to increased levels
of dopamine and subsequent improvement in the motor
symptoms of Parkinson’s disease, side effects that often result
from use of other traditional treatments relying on dopamine
replacement therapy. | | Uses | Safinamide mesylate salt has been used as a reference drug to study its inhibitory effect on human monoamine oxidases (hMAO-A and hMAO-B). | | Biochem/physiol Actions | Safinamide is a highly selective and reversible monoamine oxidase type B (MAO-B) inhibitor that increases neostriatal dopamine concentration. In addition, safinamide is voltage-dependent sodium and calcium channel blocker. It appears to bind to the batrachotoxin-sensitive site 2 of the voltage-sensitive sodium channels. Safinamide blocks N and L-type calcium channels and inhibits glutamate and aspartate release from synaptic terminals. | | Mechanism of action | Safinamide employs several mechanisms of action, functioning
as both a dopaminergic agent through inhibition of MAO-B as
well as a nondopaminergic agent via selective calcium and
sodium channel modulation, leading to inhibition of glutamate
release. At least one of several clinical studies of patients
with mid- to late-stage Parkinson’s disease showed increased
daily ON time (periods of symptom control) without
accompanying motor complications (dyskinesias) upon treatment
with safinamide, while studies of early stage Parkinson’s
disease patients treated with this drug showed significantly
improved motor symptoms during the 18-month study.
Additionally, safinamide is chemically and metabolically
stable, is well tolerated in patients, and has not exhibited
serious adverse effects even upon treatment at higher dosage
ranges. | | Pharmacology | Safinamide mesylate is a selective monoamine oxidase B inhibitor, reducing degradation of dopamine; in contrast to the other two, its action is reversible. Safinamide mesylate also inhibits glutamate release and dopamine and serotonin reuptake. It binds to the sigma receptors as well, with IC50 values for binding inhibition of 19 nM for σ1 and 1,590 nM for σ2. Additionally, it blocks sodium and calcium channels, the relevance of which for its antiparkinson action is however unknown. | | Side effects | Common adverse events in clinical trials (in more than 1% of people) included nausea, dizziness, tiredness, sleeplessness, or thostatic hypotension (low blood pressure) and headache. There was no significant difference in the occurrence of these effects between safinamide and placebo. | | Synthesis | While the reported discovery-scale synthetic approaches to
safinamide methanesulfonate were similar to the process-scale
approach, the identification of optimized and improved
reaction conditions were essential for isolation of the target in
high purity and without the presence of highly toxic
byproducts. For example, initial attempts to prepare aryl
benzyl ether 80 from benzyl chloride (78) and
phenol (79) employed conditions which led to the desired Oalkyl
product 80 in addition to the undesired C3-aryl alkylation
product, necessitating laborious and inefficient final-stage
purifications. Alternatively, employing phase transfer catalysis
conditions, specifically the use of tetradecyl trimethylammonium
bromide with K2CO3 in refluxing toluene, have become the conditions of choice, enabling
high selectivity of O-alkylation product 80 in 85% yield and
99.9% purity with minimal amounts of impurities arising from
competitive C- and O-alkylation arising after recrystallization
from diisopropyl ether. From 80, a one-pot reductive
alkylation with L-alaninamide hydrochloride 81 was effected
under standard reductive amination conditions (NaBH3CN/
MeOH). However, poor yields were observed as well as
formation of undesired byproducts. Interestingly, while not a
generally accepted method, an alternate one-pot route for
synthesis of 82 could be realized using heterogeneous reduction
conditions. Toward this end, condensation of 81 with the
aldehyde 80 was followed by immediate reduction with H2 on
wet Pt/C in MeOH, affording safinamide 82 in 92% yield
(98.4% purity). Treatment of 82 with charcoal filtration
followed by salt formation with methanesulfonic acid provided
safinamide methanesulfonate (XI) in 97% yield. In this
improved synthesis, all reactions could be performed on
multikg scale, yielding the final drug target in >99.9% purity
and containing <0.005% of the undesired C,O-bis-alkylated
derivative. 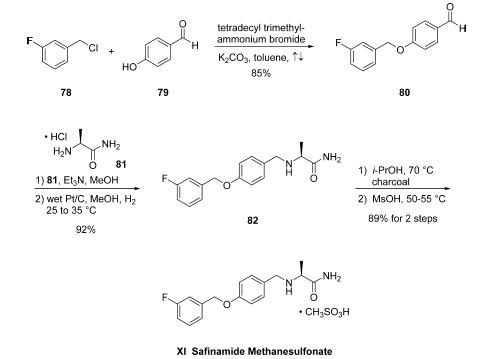
| | target | MAO-B |
| | Safinamide mesylate Preparation Products And Raw materials |
|