| | 3-NITROBENZENESULFONIC ACID Basic information |
| Product Name: | 3-NITROBENZENESULFONIC ACID | | Synonyms: | Kyselina 3-nitrobenzensulfonova;Kyselina nitrobenzen-m-sulfonova;kyselina3-nitrobenzensulfonova;kyselinanitrobenzen-m-sulfonova;m-nitro-benzenesulfonicaci;Benzene, 1-nitro-3-sulfonic acid;META-NITROBENZENESULPHONICACID;3-NITROBENZENESULFONIC ACID HYDRATE | | CAS: | 98-47-5 | | MF: | C6H5NO5S | | MW: | 203.17 | | EINECS: | 202-671-7 | | Product Categories: | | | Mol File: | 98-47-5.mol | 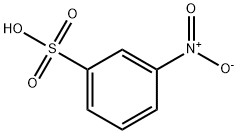 |
| | 3-NITROBENZENESULFONIC ACID Chemical Properties |
| Risk Statements | 34 | | Safety Statements | 26-36/37/39 | | RIDADR | 2305 | | RTECS | DB7190000 | | HazardClass | 8 | | PackingGroup | II | | HS Code | 2904990090 |
| | 3-NITROBENZENESULFONIC ACID Usage And Synthesis |
| Chemical Properties | Crystals. Soluble in water and
alcohol. | | Uses | Organic synthesis. The sodium salt is a protective antireduction agent. | | Uses | 3-Nitrobenzenesulfonic acid is used primarily as a mild oxidizing agent or as a precursor for colorant intermediates. In the former case it is used in processing certain anthraquinone intermediates (e.g., amination of anthraquinone-1-sulfonic acid to give 1- aminoanthraquinone) or as a dye-printing auxiliary to obtain resist effects with, for example, vat dyes. Reduction of 3-nitrobenzenesulfonic acid (usually as a liquor) to the important metanilic acid is achieved with iron or by catalytic hydrogenation. The alternative reduction with zinc and alkali or with sodium amalgam yields hydrazobenzene-3,3'-disulfonic acid, which rearranges to benzidine-2,2' - disulfonic acid [117-61-3] on treatment with acid. | | Production Methods | 3-Nitrobenzenesulfonic acid is produced by dissolving nitrobenzene in 98 % sulfuric acid and heating to 80 ℃. Then 65 % oleum is added at this temperature, and the reaction is completed by heating for 9 h at 105 ℃ (higher temperatures are unsafe). After quenching the sulfonation mass in water, neutralizing with lime, and filtering off gypsum, the calcium salt of the product is converted to the sodium salt with sodium carbonate. After calcium carbonate has been removed by filtration, the sodium salt solution can be used directly or evaporated. | | Safety Profile | A skin and severe eye
irritant. Decomposes violently at about
200°. Mixture with sulfuric acid + sulfur
trioxide may explode above 150°C. When
heated to decomposition it emits toxic
fumes of SOx and NOx. See also NITRO
COMPOUNDS OF AROMATIC
HYDROCARBONS and SULFONATES. |
| | 3-NITROBENZENESULFONIC ACID Preparation Products And Raw materials |
|