| Company Name: |
Aikon International Limited
|
| Tel: |
025-66113011 18112977050 |
| Email: |
cb6@aikonchem.com |
| Products Intro: |
Product Name:chlorthenoxazine
CAS:132-89-8
Purity:95% HPLC or GC Package:10G,5G;1G
|
| Company Name: |
Suzhou Rovathin Foreign Trade Co.,Ltd
|
| Tel: |
0512-65816829 18662214788 |
| Email: |
info@rovathin.com.cn |
| Products Intro: |
Product Name:2-(2-chloroethyl)-2,3-dihydro-4H-benzo[e][1,3]oxazin-4-one
CAS:132-89-8
Package:1g m/;10g/
|
| Company Name: |
Amel Pharmatech Corporation
|
| Tel: |
888-4366503 |
| Email: |
sales@amelpharmatech.com |
| Products Intro: |
Product Name:CHLORTHENOXAZIN(E)
CAS:132-89-8
Purity:95% Package:g; kg
|
|
| | chlorthenoxazine Basic information |
| Product Name: | chlorthenoxazine | | Synonyms: | chlorthenoxazine;chlorthenoxazin;Ap 67;Apirazin;Ossazone;Piroxina;Valmorin;Valtorin | | CAS: | 132-89-8 | | MF: | C12H8ClNO | | MW: | 217.65102 | | EINECS: | 2050823 | | Product Categories: | | | Mol File: | 132-89-8.mol | 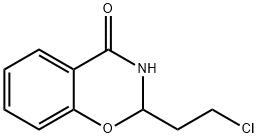 |
| | chlorthenoxazine Chemical Properties |
| Melting point | 146-147° (dec) | | Boiling point | 303°C (rough estimate) | | density | 1.2414 (rough estimate) | | refractive index | 1.5500 (estimate) | | storage temp. | Store at -20°C | | solubility | Soluble in DMSO | | pka | 12.70±0.40(Predicted) |
| | chlorthenoxazine Usage And Synthesis |
| Originator | Reugaril ,Farber ,Italy ,1966 | | Definition | ChEBI: Chlorthenoxazine is a benzoxazine. | | Manufacturing Process | A mixture of 4 liters chloroform and 1,050 cc ethanol was saturated with dry
hydrogen chloride gas at -5°C to +5°C in a vessel having a net volume of 15
liters and provided with a stirring device, reflux cooler, gas feed line,
thermometer and dropping funnel. 455 g acrolein which had been precooled
to 0°C were added dropwise to the solution over a period of 1 to 2 hours
while maintaining the temperature below +5°C and vigorously stirring. 1,070
g salicylamide and 1,080 g glacial acetic acid were added to the resulting
solution of beta-chloropropionaldehyde acetal, thereby forming a suspension
which was heated to 60°C while stirring. A clear solution was formed which
was maintained at 60°C for an additional hour. The solution was allowed to
cool to about 40°C and was then washed with water by passing a strong
stream of water under the surface of the chloroform and continuously
withdrawing the upper phase. When the water had reached a pH of 3-4, the
precipitated reaction product was separated by vacuum filtration. The
chloroform phase of the filtrate was evaporated under a weak vacuum and the
residue was combined with the precipitate first obtained. The combined
products were stirred with 2 liters of a 5% sodium hydroxide solution. The raw
reaction product was then washed with water, dried and recrystallized from
ethanol. The product had the melting point of 146°C to 147°C
(decomposition). The yield was 1,260 g, corresponding to 76% of the
theoretical yield. | | Therapeutic Function | Antipyretic, Analgesic |
| | chlorthenoxazine Preparation Products And Raw materials |
|