| Chemical Properties |
Fuming liquid.Attacks glass. |
| Uses |
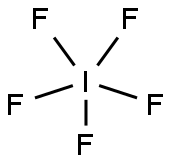
Mild fluorinating agent. |
| General Description |
A toxic colorless fuming liquid (m.p. 9° C). Decomposed by water to iodine and hydrofluoric acid. Contact with organic materials may cause their ignition. Corrosive to metals and tissue. Prolonged exposure of the container to fire or heat may result in their violent rupturing and rocketing. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. |
| Air & Water Reactions |
Fumes in air. Reaction with water or water-containing materials is violent, [Mellor 2, Supp. 1:176, 1956]. |
| Reactivity Profile |
A powerful oxidizer. Attack glass. Reacts violently with water or strong bases (potassium hydroxide, sodium hydroxide). Pentalfluoroiodide chars and usually ignites organic matter. Contact with boron, silicon, red phosphorus, sulfur, arsenic, antimony, bismuth, molybdenum and tungsten causes incandescence. Contact with potassium or sodium leads to explosions. Causes aluminum (foil, powder) to ignite. Explosive reactions with tetraiodoethylene, diethylaminotrimethylsilane. Violent reactions wilh benzene, dimethyl sulfoxide, tetraiodoethylene [Bretherick, 5th ed., 1995, p. 1434]. IF5 reacts explosively with diethylaminotrimethylsilane even at low temperature. (Oates, G. et al., J. Chem. Soc., Dalton Trans., 1974, 1383). |
| Hazard |
Dangerous fire risk, reacts violently with water. Toxic by ingestion and inhalation, corrosive to skin and mucous membranes. |
| Health Hazard |
TOXIC; inhalation or contact with vapor, substance, or decomposition products may cause severe injury or death. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. |
| Fire Hazard |
May ignite combustibles (wood, paper, oil, clothing, etc.). React vigorously and/or explosively with water. Produce toxic and/or corrosive substances on contact with water. Flammable/toxic gases may accumulate in tanks and hopper cars. Some may produce flammable hydrogen gas upon contact with metals. Containers may explode when heated. Runoff may create fire or explosion hazard. |
| Purification Methods |
Rogers et al. [J Am Chem Soc 76 4843 1954] removed dissolved iodine from IF5 by agitating with a mixture of dry air and ClF3 in a Fluorothene beaker using a magnetic stirrer. The mixture is transferred to a still, and the more volatile impurities are pumped off as the pressure is reduced below 40mm. The still is gradually heated (kept at 40mm) to remove the ClF3 before IF5 distilled. Stevens [J Org Chem 26 3451 1961] pumped IF5 under vacuum from its cylinder, trapping it at -78o, then allowing it to melt in a stream of dry N2. HARMFUL VAPOURS. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 159-160 1963.] |





 China
China