Product Details
| Product Name:
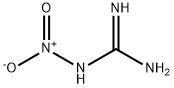
Nitroguanidine |
CAS No.:
556-88-7 |
| Min. Order:
1g |
Purity:
99% |
| Supply Ability:
100KG |
Release date:
2019/07/06 |
| PRODUCT::
AD68 |
| Nitroguanidine Basic information |
| Product Name: |
Nitroguanidine |
| Synonyms: |
LABOTEST-BB LTBB000482;[Oxido(oxo)hydrazono]methanediamine;1-nitro-guanidin;1-Nitroguanidine;2-Nitroguanidine;alpha-Nitroguanidine;beta-Nitroguanidine;Guanidine, 1-nitro- |
| CAS: |
556-88-7 |
| MF: |
CH4N4O2 |
| MW: |
104.07 |
| EINECS: |
209-143-5 |
| Product Categories: |
Guanidines;Nitrogen Compounds;Organic Building Blocks;Building Blocks;Chemical Synthesis;Nitrogen Compounds;Organic Building Blocks |
| Mol File: |
556-88-7.mol |
 |
| |
| Nitroguanidine Chemical Properties |
| Melting point |
239 °C (dec.)(lit.) |
| Boiling point |
195.15°C (rough estimate) |
| density |
1.55g/cm3 |
| refractive index |
1.6850 (estimate) |
| Water Solubility |
3 g/L (20 ºC) |
| Merck |
14,6607 |
| CAS DataBase Reference |
556-88-7(CAS DataBase Reference) |
| NIST Chemistry Reference |
Guanidine, nitro-(556-88-7) |
| EPA Substance Registry System |
Guanidine, nitro-(556-88-7) |
| Hazard Codes |
F,Xi |
| Risk Statements |
11-36/37/38-5 |
| Safety Statements |
16-26-33-36/37/39-47-37/39 |
| RIDADR |
UN 1336 4.1/PG 1 |
| WGK Germany |
1 |
| RTECS |
MF4600000 |
| HazardClass |
1.1D |
| PackingGroup |
I |
| Hazardous Substances Data |
556-88-7(Hazardous Substances Data) |
| Provider |
Language |
| Nitroguanidine |
English |
| SigmaAldrich |
English |
| ACROS |
English |
| |
| Nitroguanidine Usage And Synthesis |
| Chemical Properties |
white powder |
| Uses |
Reactant involved in the synthesis of:• ;1,5-Disubstituted-1,3,5-hexahydrotriazine-2-(N-nitro)imines with insecticidal activity1• ;Hexahydrotriazine-N-nitroimine analogs with insecticidal activity via Mannich reactions2Reactant involved in:• ;The study of the effects of vessel materials on exothermic decomposition energy3• ;Nitration of arenes4• ;Nitration of (nitrimino)tetrahyrdooxadiazine and (nitrimino)hexahydrotriazine5 |
| General Description |
Nitroguanidine is shipped as a slurry or wet mass of pale yellow crystals. If Nitroguanidine should dry out Nitroguanidine can explode due to shock, heat, flame, or friction. The primary hazard is blast where the entire load can explode instantaneously and not from flying projectiles and fragments. Under prolonged exposure to fire or heat they can explode. |
| Reactivity Profile |
Nitroalkanes, such as NITROGUANIDINE, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Nitroalkanes are insoluble in water. Flammable/combustible material. May be ignited by heat, sparks or flames. DRIED OUT material may explode if exposed to heat, flame, friction or shock; Treat as an explosive. Mercury and silver complex salts of nitroguanidine are very impact sensitive. |
| Hazard |
May explode when shocked or heated. |
| Health Hazard |
Some are toxic and may be fatal if inhaled, swallowed or absorbed through skin. Contact may cause burns to skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. |
| Safety Profile |
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A very dangerous fire hazard when exposed to heat, flame, or by chemical reaction with oxidizers. A severe explosion hazard when shocked or exposed to heat or flame. It is about as powerful as TNT. It is normally mixed with colloided nitrocellulose or ammonium nitrate and paraffin wax. Can react vigorously with oxidizing materials and the derivatives can be explosive. The mercury and silver salts and other derivatives are much more impact-sensitive. When heated to decomposition it emits highly toxic fumes of NOx. See also NITRO COMPOUNDS. |
| Purification Methods |
Crystallise it from water (20mL/g). The nitrate has m 147o(dec)( prisms, H2O). [Beilstein 3 H 126, 3 III 236.] |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
-
CAS:6363-53-7
$1.00 / 1kg
-
CAS: 108-20-3
$1.00 / 1kg
-
CAS: 37439-34-2
$1.00 / 1kg
Recommended supplier
| Product name |
Price |
|
Suppliers |
Update time |
|
|
$0.00/1KG |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-12-02 |
|
|
$0.00/1KG |
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2024-10-30 |
|
|
$15.00/1kg |
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-10-11 |
|
|
$20.00/1kg |
|
Henan Bao Enluo International TradeCo.,LTD
|
2023-08-18 |






 China
China