
Triptolide
| Price | $1 |
| Package | 1KG |
| Min. Order: | 1g |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Triptolide | CAS No.: 38748-32-2 |
| Min. Order: 1g | Purity: 99% |
| Supply Ability: 100KG | Release date: 2019/07/06 |
| Triptolide Basic information |
| Tripterygium wilfordii Anti-inflammatory and immunosuppressive effects Anti-cancer effects Using TLCS method to determine the content of triptolide |
| Product Name: | Triptolide |
| Synonyms: | PG490;TRIPTOLIDE;TRIPTOLIDE, TRIPTERYGIUM WILFORDII;,7as,7bs,8as,8bs)-;8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-,(3bs,4as,5as,6r,6ar;triptolid;TRIPTOLIDE TLC;TRIPTOLIDE95% |
| CAS: | 38748-32-2 |
| MF: | C20H24O6 |
| MW: | 360.4 |
| EINECS: | 300-006-3 |
| Product Categories: | Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Natural Anti-cancer Medical Materials and It's Derivatives;Nutritional Ingredients;Chiral Reagents;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Inhibitors |
| Mol File: | 38748-32-2.mol |
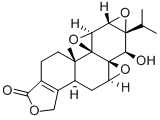 |
|
| Triptolide Chemical Properties |
| Melting point | 226-227°C |
| alpha | D25 -154° |
| Boiling point | 412.43°C (rough estimate) |
| density | 1.1656 (rough estimate) |
| refractive index | 1.4450 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble |
| form | solid |
| color | white to off-white |
| Merck | 14,9747 |
| InChIKey | DFBIRQPKNDILPW-CIVMWXNOSA-N |
| CAS DataBase Reference | 38748-32-2(CAS DataBase Reference) |
| Safety Information |
| Safety Statements | 22-24/25 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| RTECS | YK7751000 |
| HazardClass | 6.1 |
| PackingGroup | I |
| HS Code | 29321900 |
| Toxicity | LD50 i.p. in mice: 1.93 mg/kg (Chen) |
| MSDS Information |
| Provider | Language |
|---|---|
| SigmaAldrich | English |
| Triptolide Usage And Synthesis |
| Tripterygium wilfordii | There are four species under the genus Tripterygium in the world, and all are native to China, with three species being medicine. Tripterygium wilfordii is a woody climber, 2-3 meters high. Branchlets are reddish and with brown angular, with oblong tuberculate projections and iron brown hairs. Flowering period is from May to June. Fruit ripening period is from August to September. It grows in shady and humid slopes, valleys, bushes besides streams and secondary miscellaneous forests, with the distribution in the south of the Yangtze River, Anhui, Zhejiang, Fujian, Taiwan, Jiangxi, Hunan, Guangdong and even the southwest provinces. It is harvested in summer or autumn. It is bitter and poisonous, and can be used as insecticide, anti-inflammatory and detoxification. This product is poisonous, thus be careful when orally taking in. 1.Treatment of rheumatoid arthritis: smash the roots and leaves of Tripterygium wilfordii and apply it externally. Remove it half an hour later, otherwise blister could occur. (Jiangxi, " Handbook of Herbs"). 2. Treatment of skin itchiness: smash the leaves of Tripterygium wilfordii and apply it ( "Medicinal Flora in Hunan"). 3. Treatment of snake strand sore: grind the flowers of Tripterygium wilfordii and the root of three-nerved spicebush, and apply it the affected area (“ Medicinal Flora in Hunan "). |
| Anti-inflammatory and immunosuppressive effects | Triptolide is an epoxy diterpene lactone compound that extracted from the roots, leaves, flowers and fruits of Tripterygium wilfordii (Celastraceae). Triptolide, with alkaloids like wilfordine, wilforine, wilforgine, Wilfortrine, wilfozine and Wilfornine, constitutes the main active ingredient of Tripterygium extraction. It is insoluble in water, soluble in methanol, dimethyl sulfoxide, ethanol, ethyl acetate, chloroform ect. It has anti-inflammatory and immunosuppressive effects: with the application of 10μg/kg sc Triptolide in continuous 10 days, the croton oil-induced ear edema in mice can be reduced. The in vitro tests with the application of 0.05~1μg/ml Triptolide in continuous 7 days showed this product has a stabilizing effect on sheep erythrocyte membrane; with the application of 75,150mg/kgsc Triptolide in continuous 7 days, the total complement levels of rat serum significantly increased, and the formation of hemolysin antibody in primary immune response was significantly inhibited, but no significant inhibition was found in the formation of hemolysin antibody in the second immune response; with the application of 150μg/kg ig Triptolide in continuous 7 days, a certain degree of inhibition in mouse peritoneal phagocytic function; with the application of 300μg/kg sc Triptolide in continuous 15 days, the croton oil-induced ear edema in mice was inhibited; with the application of 75,150μg/kgsc Triptolide in continuous 14 days, the carrageenan paw swelling in rat was inhibited; with the application of 0.22~4.44μg/ml Triptolide in Polygalatenuifolia extraction, a protective effect on RBCM of rats was showed; with the application of 0.25,1mg/kg ip Triptolide, antibody production and secretion in mice were inhibited (QHS method); with the application of 50,500ng/ml concentration, proliferative responses of T cell and B cell were significantly inhibited; with the application of 5,50,500ng/ml Triptolide in vitro and 0.5,0.75,1mg/kg ip drug in vivo, the production of mouse spleen cells IL-2 was significantly inhibited; with the application of 0.1~1μg/ml Triptolide in vitro, Con A-induced lymphocyte growth was inhibited; with the application of 0.5~10μg/L Triptolide the mixed lymphocyte culture (MLC) was inhibited: after 60Co irradiation, the mixed lymphocyte that induced with the concentration of 5,10μg/L Triptolide inhibited the second MLC, showing that triptolide can induce suppression T cells (Ts); with the application of 0.12~0.5mg/kg ip Triptolide, the DNFB-induced DTH in mice was significantly inhibited; with the application of 0.25,0.5mg/kg Triptolide, the secretion activity of mice spleen cells IL-2 was inhibited, and the increased DTH responses that induced by high-dose cyclophosphamide was also inhibited; with the application of 0.25mg/kg Triptolide, mouse thymus Th/Ts cell ratio reduced. Experimental results indicate that triptolide has immunosuppression, and the mechanism may be related to inhibition of Th cells and secretion activity of IL-2 and inducing Ts cells. The above information is edited by the Chemicalbook of Cheng Jingmin. |
| Anti-cancer effects | In recent years, a large number of in vitro and in vivo studies demonstrated this product has good anti-tumor activity to a variety of cancers such as leukemia, breast cancer, pancreatic cancer and lung cancer. Recent studies have found that triptolide not only affects certain proteins and signaling pathways specifically, but also inhibits broad gene transcription, but the mechanism remains to be further clarified. Yu Qiang group in Shanghai Institute of Materia Medica of Chinese Academy of Sciences studied in-depth the molecular mechanisms of Triptolide in suppressing broad gene transcription. The study found that Triptolide inhibited gene transcription by promoting the phosphorylation of the largest and most important functional subunit Rpb1 in RNA polymerase II, and subsequent Rpb1 ubiquitin degradation. PTEF-b, the upstream kinases of Rpb1, plays a positive role in the regulation of phosphorylation process of Rpb1 that induced by Triptolide. The study also found that Triptolide could induce DNA damage. These results indicate that triptolide suppresses broad gene transcription by causing DNA damage in cells and thus activating the P-TEFb, which can phosphorylate Rpb1 and cause Rpb1 degradation. |
| Using TLCS method to determine the content of triptolide | 1. Chromatographic conditions: silica gel G-CMCNa plate; chloroform-ether (2: 1) as developing solvent; 2% 3,5-dinitro-benzoic acid ethanol solution and 2mol/L Sodium hydroxide ethanol solution. Mix them with the ratio 1: 3 before use as the chromogenic agent, spray the mixture and view the results under natural light. 2. The preparation of the reference solution: Weigh appropriate triptolide accurately as the reference substance, and add chloroform to make the reference solution with 1.528mg triptolide per ml. 3. Preparation of sample solution: Weigh appropriate dried herbs accurately, and make reflux extraction three times by adding ethanol, with 3 hours each time. Combine the extraction and recover ethanol to dryness, and then extract triptolide by dissolving in ethyl acetate until no triptolide presence in TLC, and recover ethyl acetate to get the extract. weigh accurately 0.4g extract powder, which has been dried at 90 ℃to constant weight and dissolve it in 5ml of dichloromethane, and mix with 8g neutral alumina, and evaporate the dichloromethane and transferre to a Soxhlet extractor, and reflux for 3h with petroleum ether (60~90 ℃), and discard the petroleum ether and remove the filter cartridge, shake off the solvent, and then reflux for 5h by placing it in an extractor with 70ml chloroform, recover chloroform to dryness and cool it, and dissolve it to precise 3ml chloroform as the sample solution. 4. Determination: precisely drawing 10μl sample solution and 2,7μl reference solution, and point them to the same piece of thin plate. According to the chromatographic conditions to spread, take out, dry and color. Cover it with the same size of glass plate and seal it for four cycles. 10min later, scan it under thin layer scanning. λS = 535nm, λR = 650nm; dual wavelength reflection zigzag scanning; slit: 1.25mm × 1.25mm; sensitivity × 5. Measure the absorption integral value of the sample and reference substance, and calculate the content by two external standard method. 5. Measurement results: Based on the above-mentioned conditions, the content of triptolide in Tripterygium root bark, root center and whole root were 0.001%, 0.0004%, 0.0018%. |
| Chemical Properties | White Crystalline Solid |
| Uses | Labelled Triptolite (T815600), diterpenoid triepoxide with immunosuppressant and antitumor properties. |
| Biological Activity | Inhibits cytokine-induced gene expression via inhibition of NF- κ B transcriptional activity. Induces apoptosis in tumor cells by blocking TNF- α mediated c-IAP2 and c-IAP1 (members of the inhibitor of apoptosis family) induction. Immunosuppressive with anti-inflammatory and antitumor activity. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $5.00/1KG |
VIP1Y
|
Wuhan JiyunZen Tech Co., Ltd.
|
2025-05-08 | |
| $0.00/25kg |
Shaanxi Haibo Biotechnology Co., Ltd
|
2023-12-05 | ||
| $30.00/1kg |
Anhui Ruihan Technology Co., Ltd
|
2023-09-07 | ||
| $50.00/1KG |
Wuhan Boyuan Import & Export Co., LTD
|
2023-04-06 | ||
| $0.00/1kg |
VIP4Y
|
Henan Aochuang Chemical Co.,Ltd.
|
2022-09-26 | |
| $90.00/1kg |
Hebei baicao biology science and technology co., ltd
|
2022-04-29 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-10 | ||
| $0.00/20mg |
Shanghai Standard Technology Co., Ltd.
|
2019-10-11 | ||
| $50.00/20mg |
NanJing Spring & Autumn Biological Engineering CO., LTD.
|
2019-07-11 | ||
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-07-08 |
- Since: 2014-12-17
- Address: Zhengzhou High tech Zone, Henan Province, China
INQUIRY
杨俊青
sales@coreychem.com
sales@coreychem.com




 China
China