
Calcium carbide
| Price | $1 |
| Package | 1KG |
| Min. Order: | 1IU |
| Supply Ability: | 100kg |
| Update Time: | 2019-12-25 |
Product Details
| Product Name: Calcium carbide | CAS No.: 75-20-7 |
| Min. Order: 1IU | Purity: 99% |
| Supply Ability: 100kg | Release date: 2019/12/25 |
| name: Cassie183 |
▼
▲
▼
▲
Product Name:
Calcium carbide
Synonyms:
ethyne,calciumderiv;Acetylenogen;ELECTROLITE;CALCIUM CARBIDE;CALCIUM ACETYLIDE;Calciumcarbidemetallurggradegreylumpsmm;carbide;CALCIUM CARBIDE, GRANULATED, 0.3-1 MM
CAS:
75-20-7
MF:
C2Ca
MW:
64.1
EINECS:
200-848-3
Product Categories:
Inorganics;Carbides;Ceramics;Metal and Ceramic Science;Non metallic mineral;inorganic chemical raw material
Mol File:
75-20-7.mol
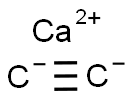
▼
▲
Calcium carbide Chemical Properties
▼
▲
Melting point
447°C
Boiling point
2300°C
density
2.22 g/mL at 25 °C(lit.)
storage temp.
water-free area
form
pieces
color
Gray-black
Specific Gravity
2.22
Water Solubility
hydrolyses
Sensitive
Moisture Sensitive
Merck
14,1656
BRN
3909011
Stability:
Stability Reacts violently with water liberating highly flammable gas (acetylene). Do not use water if this material is involved in a fire. Incompatible with moisture, water, strong oxidizing agents, alcohols, hydrogen chloride, magnesium.
InChIKey
UIXRSLJINYRGFQ-UHFFFAOYSA-N
EPA Substance Registry System
Calcium carbide (75-20-7)
▼
▲
Safety Information
▼
▲
Hazard Codes
F
Risk Statements
15-41-37/38
Safety Statements
8-43-43A-39-26
RIDADR
UN 1402 4.3/PG 2
WGK Germany
1
F
10-21
TSCA
Yes
HazardClass
4.3
PackingGroup
II
HS Code
28491000
Hazardous Substances Data
75-20-7(Hazardous Substances Data)
▼
▲
MSDS Information
▼
▲
Provider
Language
SigmaAldrich
English
▼
▲
Calcium carbide Usage And Synthesis
▼
▲
Description
Calcium carbide (molecule formula: CaC2), is a kind of important chemical raw materials produced from the chemical processing of limestone. In 1892, H. Maysan (French) and H. Wilson (United state) simultaneously developed a calcium carbide production approach based on furnace Reduction. The United State had successfully achieved industrial production in 1895. The property of calcium carbide is related to its purity. Its industrial product is mostly the mixture of calcium carbide and calcium oxide, and also contains trace amounts of sulfur, phosphorus, nitrogen and other impurities. With the increasing content of impurities, it color exhibits gray, brown to black. The melting point and electrical conductivity both decrease with the decrease of the purity. The purity of its industrial product is usually 80% with m.p. being 1800~2000 °C. At room temperature, it does not react with air, but it can have oxidation reaction at above 350 ℃, and have reaction with nitrogen at 600~700 ℃ to generate calcium cyanamide. Calcium carbide, when coming across with water or steam, generates acetylene and release a large amount of heating. CaC2 + 2H2O─ → C2H2 + Ca (OH) 2 + 125185.32J, 1kg of pure calcium carbide can produce 366 L of acetylene 366l (15 ℃, 0.1MPa). Thereby, for its storage: calcium carbide should be strictly kept away from water. It is usually packed in a sealed iron container, and sometimes stored in a dry warehouse being filled with nitrogen if necessary.


Uses
Industry
Applications
Benefit
Chemical manufacture
Production of acetylene gas
Raw materials,CaC2 + 2 H2O → C2H2 + Ca(OH)2
Production of calcium cyanamide
Raw materials, CaC2 + N2 → CaCN2 + C
Production of various acetylene derivatives
Source of acetylene gas
Production of calcium hydroxide
Raw materials, CaC2 + 2 H2O → C2H2 + Ca(OH)2
Steel production
The desulfurisation of iron (pig iron, cast iron and steel)
Desulfurization agent
As a fuel in steelmaking
Extend the scrap ratio to liquid iron
Ladle treatment facilities
A powerful deoxidizer
Mining, automobiles and street lighting
Carbide lamps
React with water to make acetylene gas, which can burn to glow
Fruit
Artificial ripening fruit
Source of acetylene gas
Signal flares
Floating, self-igniting naval signal flares
Used together with calcium phosphide
Cylinder gas
Metal fabrication and construction
Source of acetylene gas
Experiment teaching
Teaching reagent
Experiment reagent
Reaction with water
Calcium carbide will immediately have reaction upon coming across with water, generating acetylene and calcium hydroxide, which is the approach of industrial preparation of acetylene (carbide method), the reaction equation is:
CaC2 + 2H2O = C2H2 + Ca (OH) 2.
Since the impurity of calcium carbide, the generated acetylene gas is usually mixed with a small amount of hydrogen sulfide, phosphine gas and other contaminants, so there is a bad smell. Calcium carbide is produced from the lime and coke in an electric furnace at a high temperature of 3000 ℃:
3C + CaO = CaC2 + CO.
Upon the laboratory preparation of acetylene, owing to the reaction between calcium carbide and water is very fierce, we can apply saturated brine to substitute water so that a pure and smooth airflow of acetylene can be obtained. Calcium carbide won’t have reaction with sodium chloride.
CaC2 + 2H2O = C2H2 + Ca (OH) 2.
Since the impurity of calcium carbide, the generated acetylene gas is usually mixed with a small amount of hydrogen sulfide, phosphine gas and other contaminants, so there is a bad smell. Calcium carbide is produced from the lime and coke in an electric furnace at a high temperature of 3000 ℃:
3C + CaO = CaC2 + CO.
Upon the laboratory preparation of acetylene, owing to the reaction between calcium carbide and water is very fierce, we can apply saturated brine to substitute water so that a pure and smooth airflow of acetylene can be obtained. Calcium carbide won’t have reaction with sodium chloride.
Production method
Electric furnace reduction method is the only method for industrial production of calcium carbide at present. Put calcium oxide and coke for reduction reaction at 2000~2200 ℃: CaO + 3C─ → CaC2 + CO-480644.64J, the resulting molten calcium carbide flow into the receiver tank from the bottom of the reactor, and we obtain the final product after cooling. Calcium carbide production belongs to high temperature operation with relative large amount dust being produced and consuming a large amount of electrical energy. In 1980s, the production of per ton of calcium carbide consumes industrial power of about 10~11GJ. In order to reduce the power consumption, people mostly apply large-scale and closed calcium carbide furnace to reduce heat loss and also do good to the recycling of carbon monoxide.
Chemical Properties
grey or black solid with a garlic-like odour
Uses
Calcium carbide is the most relevant carbide industrially because of its important role as the basis of acetylene industry. In locations where there is shortage of petroleum, Calcium Carbide is used as the starting material for the production of acetylene (1 kg of carbide yields ~300 liters acetylene), which, in turn, can be used as a building block for a range of organic chemicals (e.g. vinyl acetate, acetaldehyde and acetic acid). In some locations, acetylene is also used to produce vinyl chloride, the raw material for the production of PVC.
A less important use of Calcium Carbide is related to the ferilizers industry. It reacts with nitrogen to form calcium cyanamide, which is the starting material for the production of cyanamide (CH2N2). Cyanamide is a common agricultural product used to stimulate early foliation.
Calcium Carbide can also be employed as desulfurizing agent for producing low-sulfur carbon steel. Also, it is used as a reducing agent to produce metals from their salts, e.g., for direct reduction of copper sulfide to metallic copper.
A less important use of Calcium Carbide is related to the ferilizers industry. It reacts with nitrogen to form calcium cyanamide, which is the starting material for the production of cyanamide (CH2N2). Cyanamide is a common agricultural product used to stimulate early foliation.
Calcium Carbide can also be employed as desulfurizing agent for producing low-sulfur carbon steel. Also, it is used as a reducing agent to produce metals from their salts, e.g., for direct reduction of copper sulfide to metallic copper.
Uses
Calcium carbide (CaC2) has a garlic-like odor and reacts with water to form acetylene gas plus calcium hydroxide and heat. In the past, it was used in miners’ lamps to continuously produce a small acetylene flame to provide some illumination in coal mines.
Preparation
Calcium carbide (CaC2) is manufactured by heating a lime and carbon mixture to 2000 to 2100°C (3632 to 3812°F) in an electric arc furnace. At those temperatures, the lime is reduced by carbon to calcium carbide and carbon monoxide (CO), according to the following reaction: CaO + 3C → CaC2 + CO
Lime for the reaction is usually made by calcining limestone in a kiln at the plant site. The sources of carbon for the reaction are petroleum coke, metallurgical coke, and anthracite coal. Because impurities in the furnace charge remain in the calcium carbide product, the lime should contain no more than 0.5 percent each of magnesium oxide, aluminum oxide, and iron oxide, and 0.004 percent phosphorus. Also, the coke charge should be low in ash and sulfur. Analyses indicate that 0.2 to 1.0 percent ash and 5 to 6 percent sulfur are typical in petroleum coke. About 991 kilograms (kg) (2,185 pounds [lb]) of lime, 683 kg (1,506 lb) of coke, and 17 to 20 kg (37 to 44 lb) of electrode paste are required to produce 1 megagram (Mg) (2,205 lb) of calcium carbide.
Lime for the reaction is usually made by calcining limestone in a kiln at the plant site. The sources of carbon for the reaction are petroleum coke, metallurgical coke, and anthracite coal. Because impurities in the furnace charge remain in the calcium carbide product, the lime should contain no more than 0.5 percent each of magnesium oxide, aluminum oxide, and iron oxide, and 0.004 percent phosphorus. Also, the coke charge should be low in ash and sulfur. Analyses indicate that 0.2 to 1.0 percent ash and 5 to 6 percent sulfur are typical in petroleum coke. About 991 kilograms (kg) (2,185 pounds [lb]) of lime, 683 kg (1,506 lb) of coke, and 17 to 20 kg (37 to 44 lb) of electrode paste are required to produce 1 megagram (Mg) (2,205 lb) of calcium carbide.
General Description
Grayish-black irregular lump solid. Used to make acetylene and in steel manufacture.
Air & Water Reactions
Reacts rapidly with water to generate the flammable gas acetylene and the base calcium hydroxide. Enough heat may be generated to ignite the gas [Jones, G.W. BM Report Invest. 3755 1944].
Reactivity Profile
Calcium carbide is a reducing agent. May react vigorously with oxidizing materials. The powdered mixture of the acetylide and iron oxide and iron chloride burns violently upon ignition, producing molten iron. Calcium carbide incandesces with chlorine, bromine, or iodine at 245, 350, or 305°C., respectively, [Mellor, 1946, Vol. 5, 862]. The carbide burns incandescently when mixed and heated with lead difluoride, magnesium, hydrogen chloride, and tin (II) chloride, [Mellor, 1946, 1940, 1946, and 1941], respectively. Interaction of Calcium carbide with methanol to give calcium methoxide is vigorous , but subject to an induction period of variable length. Once reaction starts, evolution of acetylene gas is very rapid, unpublished observations [Bretherick 1995]. Mixing Calcium carbide with silver nitrate solutions forms silver acetylide, a highly sensitive explosive. Copper salt solutions would behave similarly, [Photogr. Sci. Eng., 1966, 10, 334]. The mixture of Calcium carbide and sodium peroxide is explosive, as is Calcium carbide and perchloryl fluoride as gases at 100-300°C.
Hazard
Forms flammable and explosive gas and corrosive solid with moisture.
Health Hazard
It is a corrosive solid. Because it is highlywater-reactive, skin contact can cause burn.
Fire Hazard
Behavior in Fire: If wet by water, highly flammable acetylene gas is formed.
Safety Profile
Reaction on contact with moisture forms explosive acetylene gas. Flammable on contact with moisture, acid or acid fumes; evolves heat or flammable vapors. Moderate explosion hazard. Incandescent reaction with Cl2 (245℃), Brz (350℃), IS (305℃), HCl gas + heat, PbF2, Mg + heat. Incompatible with Se, (KOH + Ch), AgNO3, Na2O2, SnCl2, S, water. Mixtures with iron(IⅡ) chloride, iron(IⅡ) oxide, tin(Ⅱ) chloride are easily ignited and burn fiercely. Vigorous reaction with methanol after an induction period. Addttion to silver nitrate solutions precipitates the dangerously explosive silver acetylide. Copper salt solutions behave similarly. See also CALCIUM HYDROXIDE and ACETYLENE.
▼
▲
Calcium carbide Preparation Products And Raw materials
▼
▲
Preparation Products
Sodium hypochlorite-->Ethyl vinyl ether-->1,3-BUTADIENE-->Dichloroethane-->Carbon Black-->Trichloroethylene-->Polyvinyl chloride-->Acetylene-->Calcium cyanamide-->2-Butyne-1,4-diol-->Propargyl alcohol-->Cement dust-->2-Methyl-2-butanol-->Tetrapotassium hexacyanoferrate trihydrate-->Polychloroprene-->Sulfadiazine-->Difluoroethane-->VINYL CHLORIDE-->2-Butene-1,4-diol-->1,1,2,2-Tetrabromoethane-->2-Butene-1,4-diol-->1,1,2,2-TETRABROMOETHANE
Raw materials
Calcium carbonate-->Calcium oxide-->CARBON MONOXIDE-->Carbon Black-->CALCIUM CARBONATE-->METALLURGICAL COKE-->Coke (coal) -->Electrode paste
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $10.00/1KG |
VIP7Y
|
Hebei Chuanghai Biotechnology Co., Ltd
|
2025-03-13 | |
| $670.00/1T |
Yujiang Chemical (Shandong) Co.,Ltd.
|
2024-05-24 | ||
| $1.00/25kg |
Hebei Jingbo New Material Technology Co., Ltd
|
2023-11-29 | ||
| $150.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-06-09 | ||
| $10.00/1KG |
Hebei baicao biology science and technology co., ltd
|
2022-05-17 | ||
| $0.00/25KG/CTN |
VIP5Y
|
SNO CHEM MONGOLAALLIANCE
|
2021-10-19 | |
| $50.00/1KG |
Wuhan Monad Medicine Tech Co.,LTD
|
2021-04-20 | ||
| $90.00/1kg |
Hebei baicao biology science and technology co., ltd
|
2022-05-11 |
- Since: 2014-12-17
- Address: 702, Building 10, East District, National University Science and Technology Park, High tech Zone, Zh
INQUIRY






 China
China