Product Details
| Product Name: Bromhexine Impurity 20 | CAS No.: 14860-48-1 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
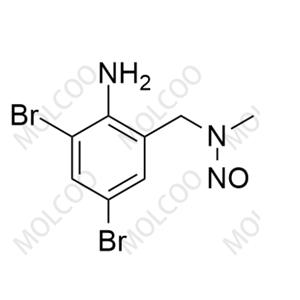
| Molecular formula: C13H18Br2N2 |
Bromhexine Impurity 20;14860-48-1
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Number: B008020
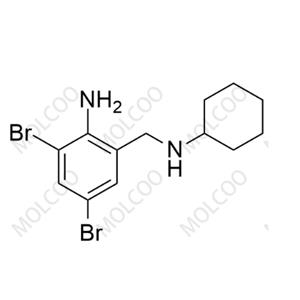
English Name: Bromhexine Impurity 20
English Alias: 2,4-dibromo-6-((cyclohexylamino)methyl)aniline
CAS Number: 14860-48-1
Molecular Formula: C₁₃H₁₈Br₂N₂
Molecular Weight: 362.10
Well-defined structure and good stability, enabling analysis of by-product formation mechanisms during bromhexine synthesis, such as bromination, amination, and alkylation reactions, to optimize processes and reduce impurity generation;
Its complex structure with a dibromo-substituted benzene ring, amino, and cyclohexylamino groups provides an accurate standard substance for detection methods like HPLC and LC-MS, significantly improving the separation and quantification accuracy of bromhexine-related impurities;
Helps study the impact of multi-functional group structures on impurity stability and potential toxicity to provide a basis for formulating scientific impurity control strategies.
Drug Development: Used as an impurity reference standard to identify and quantify Bromhexine Impurity 20 in bromhexine and its formulations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC, LC-MS), ensuring the impurity content meets pharmacopoeia and regulatory requirements during production;
Stability Studies: Investigating the degradation behavior of this impurity under light, high temperature, and high humidity conditions to evaluate its impact on bromhexine formulation stability and assist in determining storage conditions.
Detection Method Optimization: Establishing trace detection methods (detection limits reach ppb level) using techniques such as ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry (HRMS) to enhance impurity detection sensitivity in complex matrices;
Synthesis Process Improvement: Reducing impurity generation by optimizing the catalyst and reaction temperature of bromination reactions and the raw material ratio of amination reactions;
Toxicological Evaluation: Studying the potential toxicity of this impurity through in vitro cytotoxicity experiments and animal models to provide data support for formulating reasonable impurity limit standards;
Crystal Form and Physicochemical Properties: Investigating the crystal form characteristics of this impurity and its impact on the physical stability of drug formulations to improve the bromhexine quality control system
Product Number: B008020
English Name: Bromhexine Impurity 20
English Alias: 2,4-dibromo-6-((cyclohexylamino)methyl)aniline
CAS Number: 14860-48-1
Molecular Formula: C₁₃H₁₈Br₂N₂
Molecular Weight: 362.10
Well-defined structure and good stability, enabling analysis of by-product formation mechanisms during bromhexine synthesis, such as bromination, amination, and alkylation reactions, to optimize processes and reduce impurity generation;
Its complex structure with a dibromo-substituted benzene ring, amino, and cyclohexylamino groups provides an accurate standard substance for detection methods like HPLC and LC-MS, significantly improving the separation and quantification accuracy of bromhexine-related impurities;
Helps study the impact of multi-functional group structures on impurity stability and potential toxicity to provide a basis for formulating scientific impurity control strategies.
Drug Development: Used as an impurity reference standard to identify and quantify Bromhexine Impurity 20 in bromhexine and its formulations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC, LC-MS), ensuring the impurity content meets pharmacopoeia and regulatory requirements during production;
Stability Studies: Investigating the degradation behavior of this impurity under light, high temperature, and high humidity conditions to evaluate its impact on bromhexine formulation stability and assist in determining storage conditions.
Detection Method Optimization: Establishing trace detection methods (detection limits reach ppb level) using techniques such as ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry (HRMS) to enhance impurity detection sensitivity in complex matrices;
Synthesis Process Improvement: Reducing impurity generation by optimizing the catalyst and reaction temperature of bromination reactions and the raw material ratio of amination reactions;
Toxicological Evaluation: Studying the potential toxicity of this impurity through in vitro cytotoxicity experiments and animal models to provide data support for formulating reasonable impurity limit standards;
Crystal Form and Physicochemical Properties: Investigating the crystal form characteristics of this impurity and its impact on the physical stability of drug formulations to improve the bromhexine quality control system
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP8Y
|
Guangzhou PI PI BIOTECH INC
|
2021-09-04 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-11 | |
| $1980.00/50mg |
VIP2Y
|
TargetMol Chemicals Inc.
|
2025-10-27 |









 China
China