
Product Details
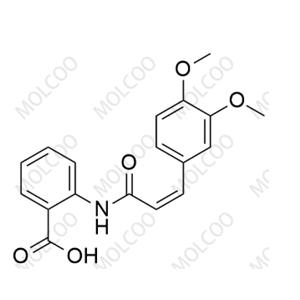
| Product Name: cis-Tranilast | CAS No.: 91920-58-0 |
| Min. Order: 1mg | Purity: >95% HPLC |
| Supply Ability: 10000 | Release date: 2025/07/31 |
| Molecular Formula:: C18H17NO5 | Molecular Weight:: 327.33 |
| Appearance: Pale yellow soild | Storage: 2-8°C Refrigerator |

| Product Catalog: | T097001 |
| CAS No.: | 91920-58-0 |
| Product Name: | cis-Tranilast |
| Purity: | >95% HPLC |
| Synonyms: | (Z)-2-(3-(3,4-dimethoxyphenyl)acrylamido)benzoic acid |
| Molecular Formula: | C18H17NO5 |
| Mol. Weight: | 327.33 |
| Appearance: | Pale yellow soild |
| Storage: | 2-8°C Refrigerator |
| Contact: | WhatsAPP: +86 17320513646 E-mail: anna@molcoo.com |
| Note: | We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS. This product is intended for laboratory use only! |
| Background: | Cis - tranilast is the cis - isomer of tranilast. Tranilast, a drug developed by Kissei Pharmaceutical Co., Ltd. (Japan) in 1982, is an anti - allergic agent used for the prevention and treatment of diseases such as allergic rhinitis, allergic asthma, and atopic dermatitis. However, the cinnamamide group in the structure of tranilast is photounstable and can be converted into cis - isomer and dimer forms when exposed to light, resulting in a decrease in bioavailability. Cis - tranilast is one of the photoisomerization products of tranilast, and its formation affects the stability and efficacy of tranilast. |
Company Profile Introduction
-
You may like
INQUIRY









 China
China