Product Details
| Product Name: Dehydro-Silodosin Impurity | CAS No.: 175870-21-0 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C25H30F3N3O4 |
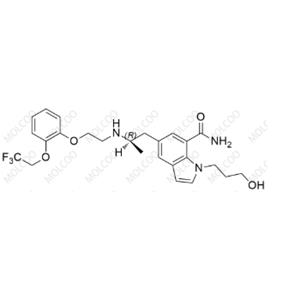
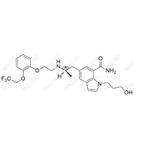
Dehydro-Silodosin Impurity
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: S013005
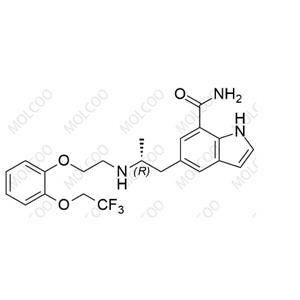
English Name: Dehydro-Silodosin Impurity
English Alias: (R)-1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)-1H-indole-7-carboxamide
CAS Number: 175870-21-0
Molecular Formula: C₂₅H₃₀F₃N₃O₄
Molecular Weight: 493.52
Advantages
Well-defined and distinct structure: Contains indole carboxamide core, trifluoroethoxyphenoxyethylamino side chain, and hydroxypropyl group, differing from silodosin by the absence of specific double bond saturation. It can be accurately identified via HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: The synergistic effect of amide and ether bonds ensures high stability, and as a by-product of incomplete hydrogenation in silodosin synthesis, it directly reflects the efficiency of reduction steps, improving the accuracy of process tracing;
High detection sensitivity: The strong electronegativity of trifluoromethyl and conjugated system of indole ring show characteristic UV absorption (270-290nm), combined with characteristic mass response (m/z 494 [M+H]⁺), enabling trace analysis via LC-MS, compatible with fluoroindole α₁ receptor antagonist detection systems.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Dehydro-Silodosin Impurity in silodosin APIs and formulations, ensuring residual dehydro impurities meet quality standards after hydrogenation;
Synthesis process optimization: Optimizing hydrogenation conditions (e.g., catalyst activity, hydrogen pressure) by monitoring impurity content to improve selectivity of target saturated products and reduce dehydro by-products;
Impurity profile enrichment: Contributing to the completeness of silodosin’s impurity profile, providing key data for comprehensive evaluation of drug purity and potential safety risks, supporting drug quality research and regulatory submissions.
Background Description
Research Status
Detection method advancement: Developing UPLC-MS/MS methods to achieve simultaneous quantification of the impurity and silodosin in complex matrices by utilizing fragment ion differences (such as characteristic cleavage of unsaturated bonds), with a detection limit as low as 0.1 ppb;
Hydrogenation mechanism analysis: Studying the formation kinetics of the impurity by simulating different catalyst types and reaction temperatures to clarify the key influencing factors of silodosin side chain hydrogenation;
Activity evaluation: Comparing the receptor affinity between this impurity and silodosin through in vitro α₁A receptor binding experiments to evaluate its potential impact on drug efficacy;
Process stability verification: Establishing a monitoring system for this impurity in large-scale production to ensure the stability and reproducibility of the hydrogenation process and reduce batch-to-batch fluctuations in impurity content
Product Information
Product Number: S013005
English Name: Dehydro-Silodosin Impurity
English Alias: (R)-1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)-1H-indole-7-carboxamide
CAS Number: 175870-21-0
Molecular Formula: C₂₅H₃₀F₃N₃O₄
Molecular Weight: 493.52
Advantages
Well-defined and distinct structure: Contains indole carboxamide core, trifluoroethoxyphenoxyethylamino side chain, and hydroxypropyl group, differing from silodosin by the absence of specific double bond saturation. It can be accurately identified via HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: The synergistic effect of amide and ether bonds ensures high stability, and as a by-product of incomplete hydrogenation in silodosin synthesis, it directly reflects the efficiency of reduction steps, improving the accuracy of process tracing;
High detection sensitivity: The strong electronegativity of trifluoromethyl and conjugated system of indole ring show characteristic UV absorption (270-290nm), combined with characteristic mass response (m/z 494 [M+H]⁺), enabling trace analysis via LC-MS, compatible with fluoroindole α₁ receptor antagonist detection systems.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Dehydro-Silodosin Impurity in silodosin APIs and formulations, ensuring residual dehydro impurities meet quality standards after hydrogenation;
Synthesis process optimization: Optimizing hydrogenation conditions (e.g., catalyst activity, hydrogen pressure) by monitoring impurity content to improve selectivity of target saturated products and reduce dehydro by-products;
Impurity profile enrichment: Contributing to the completeness of silodosin’s impurity profile, providing key data for comprehensive evaluation of drug purity and potential safety risks, supporting drug quality research and regulatory submissions.
Background Description
Research Status
Detection method advancement: Developing UPLC-MS/MS methods to achieve simultaneous quantification of the impurity and silodosin in complex matrices by utilizing fragment ion differences (such as characteristic cleavage of unsaturated bonds), with a detection limit as low as 0.1 ppb;
Hydrogenation mechanism analysis: Studying the formation kinetics of the impurity by simulating different catalyst types and reaction temperatures to clarify the key influencing factors of silodosin side chain hydrogenation;
Activity evaluation: Comparing the receptor affinity between this impurity and silodosin through in vitro α₁A receptor binding experiments to evaluate its potential impact on drug efficacy;
Process stability verification: Establishing a monitoring system for this impurity in large-scale production to ensure the stability and reproducibility of the hydrogenation process and reduce batch-to-batch fluctuations in impurity content
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $31.00/10mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-12-03 | |
| $100.00/50kg |
VIP2Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-13 | |
| $50.00/1kg |
VIP4Y
|
Hebei Dangtong Import and export Co LTD
|
2023-10-09 |









 China
China