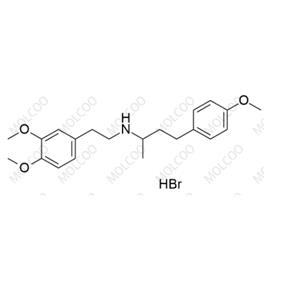
Product Details
| Product Name: Dobutamine EP Impurity C(Hydrobromide) | Min. Order: 10mg |
| Purity: 99%+ HPLC | Supply Ability: 1000 |
| Release date: 2025/07/31 | |
| Molecular formula: C21H29NO3.HBr |
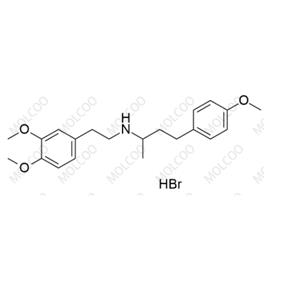
Dobutamine EP Impurity C(Hydrobromide)
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: D044002B
English Name: Dobutamine EP Impurity C(Hydrobromide)
English Alias: N-(3,4-dimethoxyphenethyl)-4-(4-methoxyphenyl)butan-2-amine hydrobromide
CAS Number: None
Molecular Formula: C₂₁H₂₉NO₃·HBr
Molecular Weight: 424.37(343.46 + 80.91)
Advantages
Well-defined structure and stable salt form, enabling analysis of by-product formation mechanisms during dobutamine synthesis, such as benzene ring substitution and amination reactions, to optimize processes and control impurity generation;
As a salt-form reference standard containing polymethoxybenzene rings and amino groups, it provides a stable quantitative standard for HPLC, LC-MS, and other detection methods, improving method accuracy;
The hydrobromide form enhances water solubility, facilitating simulation of impurity behavior in physiological environments during formulation stability studies.
Applications
Drug Development: Used as an impurity reference standard to identify and quantify Impurity C in dobutamine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC or LC-MS), ensuring compliance with EP pharmacopoeia requirements;
Stability Studies: Investigating the degradation behavior of hydrobromide under acidic/alkaline conditions to evaluate its impact on dobutamine injection stability.
Background Description
Research Status
Detection Method Optimization: Establishing trace detection methods using UPLC-MS/MS technology, leveraging the ionization characteristics of hydrobromide to enhance mass spectrometry response sensitivity;
Synthesis Process Improvement: Reducing by-product generation by optimizing the catalyst (such as palladium carbon) and reaction temperature of methoxylation reactions;
Salt Form Stability: Studying the hygroscopicity and degradation pathways of hydrobromide under high temperature and humidity conditions to provide data for storage conditions;
Toxicological Evaluation: Evaluating the potential impact of this impurity on cardiac muscle cells through in vitro cytotoxicity experiments to assist in setting safe limits
Product Information
Product Number: D044002B
English Name: Dobutamine EP Impurity C(Hydrobromide)
English Alias: N-(3,4-dimethoxyphenethyl)-4-(4-methoxyphenyl)butan-2-amine hydrobromide
CAS Number: None
Molecular Formula: C₂₁H₂₉NO₃·HBr
Molecular Weight: 424.37(343.46 + 80.91)
Advantages
Well-defined structure and stable salt form, enabling analysis of by-product formation mechanisms during dobutamine synthesis, such as benzene ring substitution and amination reactions, to optimize processes and control impurity generation;
As a salt-form reference standard containing polymethoxybenzene rings and amino groups, it provides a stable quantitative standard for HPLC, LC-MS, and other detection methods, improving method accuracy;
The hydrobromide form enhances water solubility, facilitating simulation of impurity behavior in physiological environments during formulation stability studies.
Applications
Drug Development: Used as an impurity reference standard to identify and quantify Impurity C in dobutamine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC or LC-MS), ensuring compliance with EP pharmacopoeia requirements;
Stability Studies: Investigating the degradation behavior of hydrobromide under acidic/alkaline conditions to evaluate its impact on dobutamine injection stability.
Background Description
Research Status
Detection Method Optimization: Establishing trace detection methods using UPLC-MS/MS technology, leveraging the ionization characteristics of hydrobromide to enhance mass spectrometry response sensitivity;
Synthesis Process Improvement: Reducing by-product generation by optimizing the catalyst (such as palladium carbon) and reaction temperature of methoxylation reactions;
Salt Form Stability: Studying the hygroscopicity and degradation pathways of hydrobromide under high temperature and humidity conditions to provide data for storage conditions;
Toxicological Evaluation: Evaluating the potential impact of this impurity on cardiac muscle cells through in vitro cytotoxicity experiments to assist in setting safe limits
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $790.00/500mg |
VIP2Y
|
R&D Scientific Inc.
|
2025-11-25 | |
| $0.00/10mg |
VIP8Y
|
Guangzhou PI PI BIOTECH INC
|
2022-04-25 | |
| $0.00/10mg |
VIP8Y
|
Guangzhou PI PI BIOTECH INC
|
2021-09-19 |








 China
China