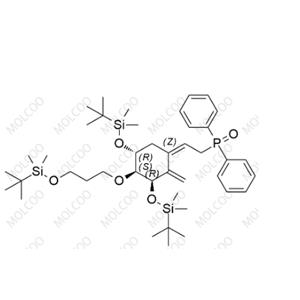
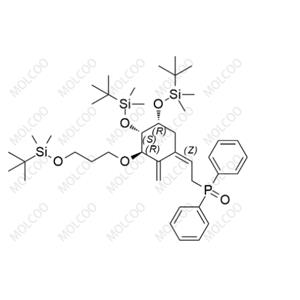
Eldecalcitol Impurity20;1101133-78-1

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: E034020
English Name: Eldecalcitol Impurity 20
English Alias: ((Z)-2-((3R,4S,5R)-3,5-bis((tert-butyldimethylsilyl)oxy)-4-(3-((tert-butyldimethylsilyl)oxy)propoxy)-2-methylenecyclohexylidene)ethyl)diphenylphosphine oxide
CAS Number: 1101133-78-1
Molecular Formula: C₄₂H₇₁O₅PSi₃
Molecular Weight: 771.24
As an impurity of Eldecalcitol, this compound has the following advantages:
Well-defined with prominent functional groups: Contains three tert-butyldimethylsilyl (TBS) groups, diphenylphosphine oxide (DPPO), (Z)-ethylidene side chain, and propoxy moieties. Combined hydrophobicity of silyl groups and polarity of phosphine oxide enable distinct retention behavior from eldecalcitol, allowing accurate identification via normal-phase HPLC/LC-MS as a specific impurity marker;
High stability and traceability: TBS-mediated hydroxyl protection and chemical stability of phosphine oxide ensure stability across wide pH ranges. As an intermediate from residual phosphine-coupling reagents, incomplete desilylation, or blocked cyclization, it directly reflects efficiency of phosphine-involved steps, improving process tracing accuracy;
High detection sensitivity: Strong UV absorption (250-270nm) from (Z)-alkene-benzene conjugation, combined with phosphorus isotope (³¹P) and silicon-specific mass responses (e.g., m/z 772 [M+H]⁺), enables trace analysis (ppb level) via LC-MS/MS, compatible with silyl-protected/phosphine-containing vitamin D precursor systems.
Pharmaceutical quality control: Used as an impurity reference standard to quantify Eldecalcitol Impurity 20 in APIs, ensuring residual phosphine/silylated intermediates meet quality standards post-phosphine coupling/desilylation/cyclization;
Synthesis optimization: Optimizing DPPO reagent dosage, coupling reaction conditions (temperature, time), and TBS deprotection by monitoring impurity levels to enhance alkene formation efficiency;
Intermediate purity assessment: Evaluating purity of key phosphine-substituted alkene intermediates in eldecalcitol synthesis to support specificity of downstream cyclization/oxidation.
Eldecalcitol synthesis often relies on phosphine reagent (e.g., diphenylphosphine oxide derivatives) coupling reactions to construct alkene structures. Unreacted phosphine reagents, incomplete TBS deprotection, or blocked cyclization may generate DPPO-containing/silylated derivatives like Eldecalcitol Impurity 20. Its DPPO group risks interfering with metal-catalyzed steps and lacks bioactivity, making control critical for eldecalcitol quality assurance.
Current research focuses on:
Analytical method validation: Developing UPLC-MS/MS assays with phosphorus fragment monitoring (e.g., m/z 201 [Ph₂PO]⁺) for simultaneous quantification of impurity and intermediates, achieving 0.1 ppb detection limits;
Coupling reaction kinetics: Studying impurity formation under varying phosphine reagent concentrations to clarify byproduct formation mechanisms in phosphine-involved coupling;
Process refinement: Controlling impurity levels below 0.05% via optimized coupling parameters to enhance API purity;
Structural characterization: Using ³¹P-NMR and 2D-NMR to confirm (3R,4S,5R) configuration and (Z)-geometry, supporting structural differentiation from eldecalcitol precursors
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com










 China
China