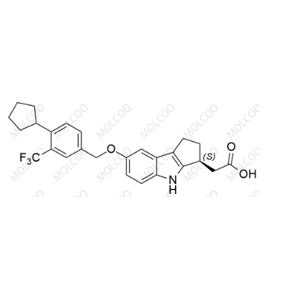
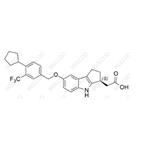
Product Details
| Product Name: Etrasimod Impurity 21 | CAS No.: 1206123-51-4 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
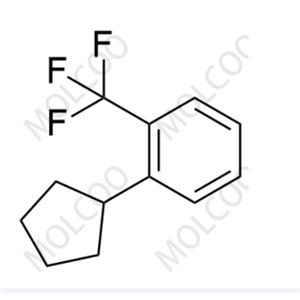
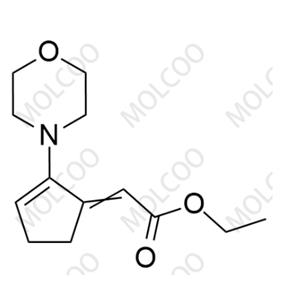
Etrasimod Impurity 21;1206123-51-4
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!
Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $48.00/1mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-12-10 | |
| $5.00/1KG |
VIP1Y
|
Wuhan JiyunZen Tech Co., Ltd.
|
2025-06-05 | |
| $0.00/1g |
VIP2Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-06-28 |
INQUIRY








 China
China