Evocalcet Impurity21;870859-14-6
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: E056021
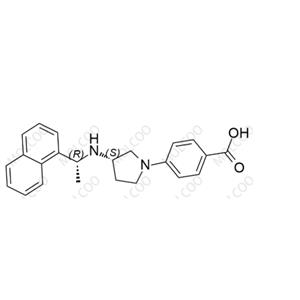
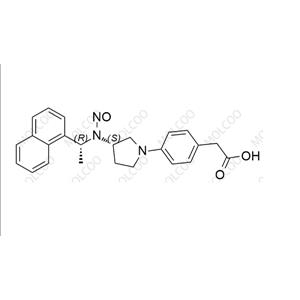
English Name: Evocalcet Impurity 21
English Alias: 4-((S)-3-(((R)-1-(naphthalen-1-yl)ethyl)amino)pyrrolidin-1-yl)benzoic acid
CAS Number: 870859-14-6
Molecular Formula: C₂₃H₂₄N₂O₂
Molecular Weight: 360.45
As an impurity of Evocalcet, this compound has the following advantages:
Well-defined and chiral-specific structure: Contains (S)-pyrrolidine, (R)-1-(naphthalen-1-yl)ethylamino, and benzoic acid groups, with chiral configurations (S/R) closely related to evocalcet. Conjugation of benzene and naphthalene rings enables unique polarity and retention, allowing accurate identification via chiral HPLC and LC-MS as a specific impurity marker;
High stability and traceability: Tertiary amine in pyrrolidine and carboxylic acid in benzoic acid ensure stability under neutral to weakly acidic conditions. As an intermediate derivative from chiral amine condensation in evocalcet synthesis, it directly reflects chiral center construction efficiency, improving process tracing accuracy;
High detection sensitivity: Strong UV absorption (270-290nm) from naphthalene-benzene conjugation, combined with characteristic mass response (m/z 361 [M+H]⁺), enables trace analysis via LC-MS (ppb level), compatible with calcimimetic impurity systems.
Pharmaceutical quality control: Used as an impurity reference standard to quantify Evocalcet Impurity 21 in APIs, ensuring residual chiral impurities from amine condensation meet quality standards;
Synthesis optimization: Optimizing condensation of (S)-pyrrolidine and (R)-1-(naphthalen-1-yl)ethylamine (catalyst, temperature) by monitoring impurity levels to enhance chiral selectivity;
Chiral purity assessment: Evaluating configurational purity of key chiral intermediates in evocalcet synthesis to support salt formation and purification efficiency.
Evocalcet, a calcimimetic for secondary hyperparathyroidism, features chiral pyrrolidine and naphthylethylamino groups. During synthesis, incomplete condensation of (S)-pyrrolidine with (R)-1-(naphthalen-1-yl)ethylamine or chiral racemization may generate chiral derivatives like Evocalcet Impurity 21. Its differing calcimimetic activity and potential metabolic impacts make control critical for evocalcet quality assurance.
Current research focuses on:
Analytical method validation: Developing UPLC-chiral column assays with mass detection for enantioseparation of impurity and evocalcet, achieving 0.1 ppb detection limits;
Chiral condensation kinetics: Studying impurity formation under varying chiral catalyst conditions to clarify stereoselectivity factors in chiral center construction;
Chiral stability studies: Evaluating racemization rates under accelerated storage to guide formulation design for maintaining optical purity;
Bioactivity comparison: Assessing differences in calcium-sensing receptor activation between impurity and evocalcet to establish scientifically based impurity limits.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com









 China
China