Exatecan Impurity
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Information
Product Number: E068006
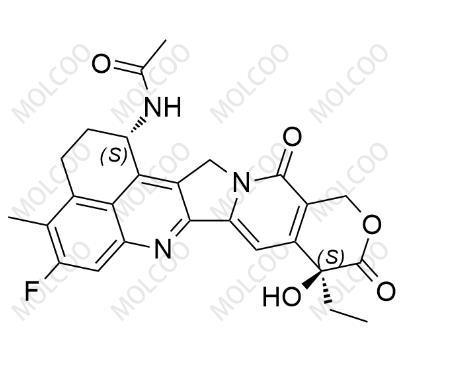
English Name: Exatecan Impurity 6
English Alias: N-((1S,9S)-9-ethyl-5-fluoro-9-hydroxy-4-methyl-10,13-dioxo-1,2,3,9,10,12,13,15-octahydrobenzo[de]pyrano[3',4':6,7]indolizino[1,2-b]quinolin-1-yl)acetamide
CAS Number: 2922852-48-8
Molecular Formula: C26H24FN3O5
Molecular Weight: 477.48
Advantages: As a reference standard for Exatecan Impurity 6, its structure is confirmed by multiple methods including nuclear magnetic resonance (NMR), mass spectrometry (MS), and infrared spectroscopy (IR), with a purity of not less than 98.5% (HPLC normalization). Under storage conditions of 2 - 8°C in the dark and sealed, it exhibits stable chemical properties, with a shelf life of up to 24 months and excellent batch-to-batch quality consistency. It can be precisely used for the analysis of related impurities of exatecan, providing a reliable basis for drug quality control.
Applications:
Impurity Detection: Used to establish HPLC and LC - MS detection methods for Impurity 6 in exatecan bulk drugs and formulations, determine the limit of detection and limit of quantification, and control the impurity content to meet international standards such as ICH Q3A.
Process Optimization: During the production of exatecan, monitor the content of Impurity 6 in real time. Analyze the impact of factors such as reaction temperature, time, and raw material ratio on its generation, optimize the synthesis process, reduce impurity generation, and improve product quality.
Stability Research: In drug accelerated stability tests (such as 60℃/RH75%) and long-term stability tests, track the change trend of Impurity 6 content, evaluate the quality stability of drugs under different storage conditions, and provide data support for determining the shelf life and storage conditions of drugs.
Regulatory Compliance: Assist pharmaceutical companies in meeting the strict requirements of domestic and international drug regulatory agencies such as FDA, EMA, and NMPA for drug impurity limits. Provide accurate impurity detection data during drug registration and application to ensure that products meet regulatory standards.
Background Description: Exatecan is a topoisomerase I inhibitor mainly used for cancer treatment. Due to the complexity of its synthesis process involving multiple chemical reactions and raw material characteristics, various impurities are likely to be generated, and Impurity 6 is one of them. The presence of such impurities may affect the safety and effectiveness of the drug. With the increasingly strict requirements of the global drug regulatory system for drug impurity control, the research and control of Exatecan Impurity 6 have become a key link in ensuring drug quality and patient medication safety.
Research Status:
Detection Technology: Currently, LC - MS/MS technology is widely used to detect Impurity 6. By optimizing the chromatographic column (such as C18 column, 1.8μm particle size), the mobile phase system (gradient elution of acetonitrile - 0.1% formic acid aqueous solution), and mass spectrometry parameters, highly sensitive detection of Impurity 6 can be achieved, with a detection limit as low as 0.01 ng/mL, significantly improving the detection accuracy compared with traditional HPLC methods.
Formation Mechanism: Research has found that Impurity 6 may originate from side reactions of acetylation or cyclization during the synthesis of exatecan. Trace impurities in raw materials or improper control of reaction conditions (such as temperature fluctuations, catalyst residues) will promote its generation. By improving reaction conditions and optimizing purification steps, the generation of Impurity 6 can be effectively inhibited.
Safety Evaluation: The toxicological research on Impurity 6 is gradually progressing. Preliminary in vitro cell experiments show that high concentrations of Impurity 6 have a certain impact on cell activity, but the specific toxic mechanism is still unclear. At present, strict limit requirements have been set for it in drug quality standards. In the future, more animal experiments and pre-clinical studies are needed to further explore its toxic characteristics and improve impurity control strategies.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!












 China
China