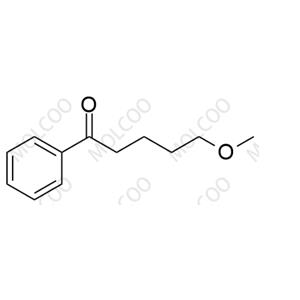
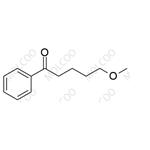
Product Details
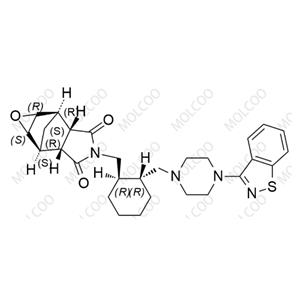
| Product Name: Fluvoxamine Impurity 14 | CAS No.: 34008-70-3 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C12H16O2 |
Fluvoxamine Impurity 14;34008-70-3
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $32.00/2mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-11-04 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-26 | |
| $0.00/1kg |
VIP5Y
|
Sinoway Industrial co., ltd.
|
2024-06-25 |
INQUIRY








 China
China