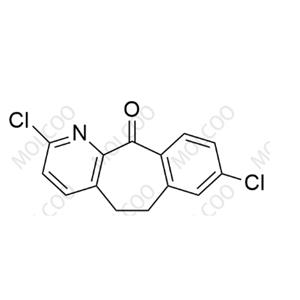
Product Details
| Product Name: Loratadine Impurity | CAS No.: 133330-61-7 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
Loratadine Impurity

Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $/ |
VIP5Y
|
RongNa Biotechnology Co.,Ltd
|
2025-04-29 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-09 | |
| $0.00/1Kg/Bag |
VIP5Y
|
Sinoway Industrial co., ltd.
|
2023-06-01 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-05-28 |
INQUIRY








 China
China